The potential of three-dimensional printing technologies to unlock the development of new ‘bio-inspired’ dental materials: an overview and research roadmap
Abstract
Purpose: Bio-inspiration is an approach in engineering aimed at optimizing artificial systems by borrowing biological concepts from nature. This review sets out to summarize the fundamental aspects employed by nature to avoid premature dental failures. On the basis of these findings, it then defines and evaluates rules for ‘post-modern’ manufacturing processes to imitate or regenerate complex biological systems. Further details about digital technologies in dentistry are accessible for you to learn on our website in Digital Dentistry section.
Study selection: A thorough literature search was conducted using PubMed, the Cochrane Library database and Google Scholar. Peer-reviewed articles and other scientific literature provided up-to-date information addressing two topics: (a) how natural dental tissues combine to create a structure as tough, strong and highly resistant to fatigue failure as tooth, and (b) how ‘bio-inspiration’ can be applied to the manufacture of dental restorations,taking into consideration the limitations of techniques currently used in dentistry.
Results: Bio-inspired concepts have already been successfully applied in a range of engineering fields to enhance the toughness and strength of artificial materials. The area of technology with greatest potential to unlock the development of these new approaches is additive manufacturing. Consequently, these technologies and concepts could be applied to dentistry to improve the mechanical properties of dental restorations. Three-dimensional (3D) printing technologies also offer a new and promising prospect of regenerating dental tissues.
Conclusions: Considering the limitations to both conventional and subtractive computer-aided design/ computer-aided manufacturing (CAD/CAM) methods, further research should focus on new, additive 3D printing techniques. This may open new research paths in dentistry that will enhance the clinical performance of artificial dental materials.
1. Introduction
Over billions of years of evolution, nature has invented many ingenious solutions to many complex problems. Today’s cutting-edge technologies have since converted nature into a source of inspiration, allowing the extrapolation and application of its different approaches to attempt to solve contemporary engineering problems. The process has become increasingly common in scientific research and has received a variety of labels in recent years: biomimetics, bio-inspiration, bionics, biomimicry, nature-based solutions, etc. The engineer and physicist Schmitt OH, first used the term ‘biomimetics’ in 1957 to describe a biological approach to engineering [1,2]. In 1974, Webster’s Dictionary added its first definition of ‘biomimetics’: “the study of the formation, structure, or function of biological materials, mechanisms and processes in order to synthesize artificial products that mimic natural ones.” Nowadays, under International Organization for Standardization (ISO) standard no.18458:2015 [3], ‘biomimetics’ is defined as the “ . . . interdisciplinary cooperation of biology and technology or other fields of innovation with the goal of solving practical problems through the function analysis of biological systems, their abstraction into models, and the transfer into and application of these models to the solution.” Nevertheless, there is nothing to forbid the labelling of any technological development as ‘bio-inspired,’ just so long as there is convincing evidence of an existing biological model generator [1]. This translation of information from natural models (such as biological materials, functions, structures or processes) into artificial devices has opened up the possibility of developing novel research strategies that incorporate 3.8 billion years of evolution [4].
The process starts with the analysis of a biological system and the understanding of its function within a hierarchical architecture and micro-/nano-structure, and then progresses to the identification of the determining concept in the success of that system and the synthesis of a model that is synthetically reproducible and typically less complex than its biological counterpart [5,6]. In many instances, it is still futile to attempt to recreate a biological composite exactly: even with today’s advanced technology, the replication of complex micro-/nano-structures, such as those observed in biological composites like in bone or nacre, is not yet possible. The end goal of ‘biomimetics’ remains, however, and current methods continue to approach it by drawing upon disciplines such as biology, physics and technology. Without doubt, it is only by using an interdisciplinary approach, and thereby associating not only different methods but also different ways of thinking, that it will become possible to solve technical problems through the abstraction, transfer and application of knowledge obtained from biological models [7]. Nature has implemented myriad strategies and thus demonstrated its ability to solve countless functional problems, and therefore bio-inspiration essentially constitutes a limitless source of new ideas [8]. One subject that fascinates scientists is the exceptional physical performance of the complex architectures and structures found in natural composite materials. For example, some natural hard tissues show combined physical properties that exceed the sum of the individual components by orders of magnitude [9,10].
The nacre of a mollusk shell, for example, mostly consists of a fragile mineral component, yet, despite the expectation that this structure would display a highly brittle mechanical behavior – given its high content of minerals – it is actually 3000 times tougher than the minerals of which it is made. Nacres are therefore damage tolerant and physically tough: they can even produce ‘quasi-ductile’ behavior and absorb an exceptional amount of deformation [11,12]. Another example is mammalian teeth, which are remarkably resilient structures [13]. Even though they are composed of only brittle mineral and weak organic phase, they are able to tolerate the high forces generated by a person chewing thousands of times a day [14].
The main purpose of this review is to summarize the fundamental aspects employed by nature in its toughening mechanism for teeth, thereby allowing the definition and critical analysis of those rules with a view to establishing modern manufacturing processes capable of replicating complex systems. In the light of the technical limitations to both classical and computer-aided subtractive manufacturing methods, the development and fine-tuning of new devices based on additive manufacturing is proposed. This may open new research paths in the area of dental materials, leading to enhanced mechanical and esthetic properties.
2. Natural tooth model
Natural tooth shows mechanical behaviors that outperform those of its constituents and the properties of their homogeneous mixtures [10,12]. This performance is possible thanks to the natural strategy of creating a hierarchical architecture made of interwoven or interlocking structures at different dimensional levels (nano-, micro- and macro-scales) comprising every component of the tooth. In fact, teeth are composed of two distinct hard components: an external hard shell, the enamel, and an internal tough core, the dentin. The transition area between them is known as dentino–enamel junction (DEJ) [15].
Table 1. Mechanical properties of enamel and dentin. The wide range of values is a reflection of the many experimental variables at play: (a) type of mechanical test used; (b) environmental condition of specimens (humidity, temperature); (c) anatomical variation of specimens (tubule orientation, dimension and density in dentin; prism and tuft arrangement in enamel); (d) orientation of stress applied and location of tested structure in the tooth. In terms of spatial distribution, external enamel shows the highest brittleness, hardness and elastic modulus. Mantle dentin and external, tubule-poor dentin are harder, more brittle and less elastic than inner, tubule-rich dentin. Within the dentino-enamel junction (DEJ), mechanical properties gradually shift from enamel to dentin.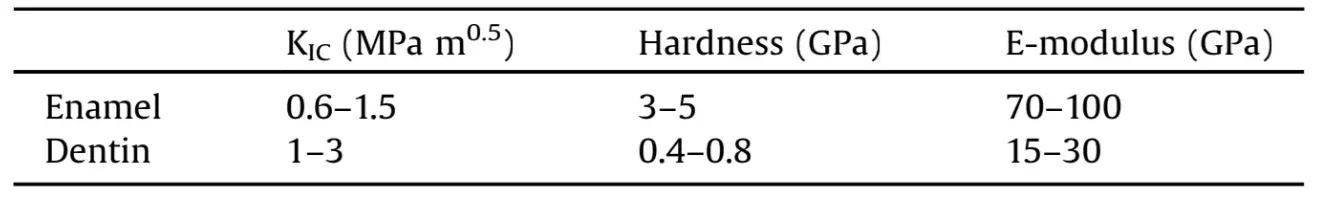
Tooth enamel is a hard, extracellular tissue with 96 vol% mineral phase 4 vol% organic material and water [16,17], displaying hierarchical organization from the nano- to the macro-scale [18,19]. The fundamental blocks of this organization are nano-scale crystals of carbonate hydroxyapatite (d. 25 nm, w. 100 nm and l. >100 nm), which are predominantly aligned along their longitudinal axes and glued together by proteins. The union of these crystals form nanofibers, which represent the first hierarchical level of organization. These fibers are bundled together into rod-like structures, known as ‘prisms’ (l. 0.1–1.5 mm, dia. 5 mm), and into the interprismatic matrix [20,21]. Prisms constitute the microscale building blocks, the second hierarchical level of organization of enamel. At the boundary between the prisms and interprismatic substance there is an ultra-thin (<1mm) sheath-like structure known as enamel ‘tufts,’ composed of a non-collagenous, organic matrix [22,23]. The third level of hierarchical organization is determined by the arrangement and orientation of the prisms: they are mostly parallel between tufts and perpendicular to the tooth surface in the outer region of the enamel. Deep inside the enamel, they are decussated and crisscross each other, forming sinusoidal pathways [24].
The DEJ is an interlinking zone that measures 25–100mm in thickness and is characterized by a cross-sectional scalloped profile [25]. This arrangement increases the surface area of the interface between the two biological materials, allowing for a strong bond [26]. The DEJ also ensures a soft and gradual transition between not only the components of enamel and dentin, but also their mechanical properties (Table 1) [27–40].
Dentin forms the bulk of the tooth and is a hard tissue composed of 45 vol% inorganic material, 33 vol% organic material and 22 vol% water [15]. The architecture is organized mainly around ‘dentinal tubules.’ These tunnels of 1–2.5mm diameter across the whole width of the dentin are traced by the odontoblast processes during dentinogenesis. A highly calcified collagen- lacking matrix, known as the peritubular dentin (PTD), surrounds the dentinal tubules and is itself embedded in a softer and collagen-rich (Type I) matrix, called the intertubular dentin (ITD). A semi-membranous electron-dense sheet-like structure, called the lamina limitans, divides the PTD and the ITD throughout its entire length [41,42]. The lamina limitans is chiefly composed of non-collagenous proteins, such as proteoglycans (PGs), glycosaminoglycans (GAGs) and phosphorylated molecules [43–45], which appear to play an important role in the mechanical behavior of dentin. In fact, GAGs and PGs regulate hydrostatic and osmotic pressure through their high hydrophilicy; and their selective removal, by enzymatic dissolution, results in a drop of E-modulus and hardness of the dentinal tissue [46–48].
Fractures of natural dental materials supposedly occur all of a sudden at a critical tensile stress. Having analyzed the fracturing of teeth, however – from a superficial fissure to a catastrophic splitting – it is evident that the strategy used by nature to ensure the survival of teeth follows much more complex rules. Although the hard and brittle enamel shell protects the underlying dentin from wear damage, it is not resistant to fissures, even though it does contain them [49]. In fact, its design acts efficiently to enclose any precursor cracks and thus prevent a clear threat to the bulk integrity of the tooth [50].
Closer examination of these superficial fractures reveals a range of different kinds of cracks in the enamel: (a) ‘median’ and ‘cone’ cracks start from the contact point and show a descending propagation [51]; (b) ‘margin’ cracks start from the cervical area and show an ascending propagation [52]; and (c) ‘radial’ cracks start from the inner DEJ and also show an ascending propagation [53].
Tufts are believed to be the main cause of most of the radial upward partial fissures [54]. These visible, crack-like defects initiate easily in enamel as a consequence of function or overload and subsequently they stabilize at different levels throughout the enamel [55]. This mechanism supported by tufts provides an internal shielding effect from occlusal stresses. In fact, even though those defects are easy to induce, they hardly evolve during a catastrophic failure of a tooth. On the contrary, the resistance results increase as any cracks are filled with organic fluids, “gluing” the crack walls together (self-healing) [14]. Even though their role has been underestimated for a long time, tufts do provide a stabilizing and toughening effect without which enamel would be more brittle than glass.
Downward fractures are channeled through the parallel arrangement of the prisms and along the interprismatic substance, protecting the enamel surface from chipping [56,57]. Crack diffusion is impeded by the decussation pattern of the third hierarchical level of the inner enamel, which contributes to the induction of crack deflection, crack bifurcation and crack bridging [20,58]. These toughening mechanisms contribute to increasing the crack-growth resistance of enamel. Nevertheless, if the applied stress increases consistently, it may induce a transmission of the fracture down to the DEJ, a more complex microarchitecture that acts as a shield and prevents further crack growth [59]. At this level, the crack can be halted: the energy of the propagation is either absorbed by the tougher layer of mantle dentin or deviated in a manner to produce a spalling of the enamel away from the dentin [14,51]. The progression of a fatigue crack through the dentinal tissue is considered uncommon, but a particularly high overload can cause the fracture to spread throughout the bulk of the tooth [60]. Transverse cracks can spread into the dentin causing the loss of a large portion of the tooth. Catastrophic failure may also occur when longitudinal cracks cause the entire tooth to split [61].
The evolutionof a crackishighlyinfluenced bythemicrostructure of dentin [62,63]. In fact, fractures are prone to interact with microscopic features, flanking them or going through them, and following weaker interfaces, thereby generating tortuous paths. The orientation, density and dimensions of dentinal tubules are part of the toughening mechanisms of dentin as they increase its damage tolerance: crack deflection, ligament bridging and crack branching are the most important and efficient toughening aspects [64]. All these mechanisms are activated when a crack first occurs, resulting in the rising resistance-curve (R-curve) behavior of the tissue as the crack expands. Wide tubules are more prone to initiate these toughening mechanisms [65]. Conversely, the emergence and increasing number of fracture-filled tubules due to age-related degradationincreases the brittleness ofdentin[66].Moreover, when the crack direction runs parallel to the tubules, its progression is promoted when compared to the perpendicular direction [67].
The appearance of a tooth is related to the optical properties of its histo-anatomic structure, its three-dimensional configuration and the respective internal, spatial relationships of its main constituents: dentin and enamel [68]. Dental enamel is an almost colorless and translucent tissue. Unlike glass, which is transparent, it can refract and scatter lightthrough its microstructure producing different optical effects [69].
Enamel shows optical anisotropy, which is indicative of the predominant role played by the rods in the propagation of light [70,71]. The opalescent properties of enamel are mainly determined by the arrangement and size of the hydroxyapatite crystals and rods, which are able to scatter only a few short wavelengths in the blue range and to transmit only longer wavelengths in the red range [72,73]. Accordingly, teeth display a bluish translucency, especially around the incisal edge, when observed from outside the mouth, and a reddish/orange appearance from the inside the mouth. The opacity of dentin is more than three times higher than that of enamel, and its saturation and brightness influence the amount of light reflected [74]. In fact, the color of a tooth depends mainly on the color of dentin [75,76]. There is a gradation of color (and translucency) in different areas of a tooth. Starting from the cervical area, there is a more saturated chroma and less translucency; toward the incisal edge, the saturation decreases and the translucency becomes more evident. In some individuals, however, such as adults and the elderly, an inverse graduation may be observed. Tooth color changes over the lifetime of an individual [77–79] due to multiple reasons. On the one hand, enamel thickness decreases as a consequence of physiological wear. As a result of this, the chroma of dentin becomes more evident. As dentin ages, it becomes thicker due to the deposition of tertiary dentin. Meanwhile, the microstructure of the dentin is also altered, especially close to the DEJ. This change makes teeth darker and more yellowish. In summary, in order to understand the strategies that nature uses to avoid premature dental failure, an in-depth understanding of the structure of teeth at micro- and nano-scale is needed (Fig.1).
Biological structures such as the mammalian teeth exhibit optimized biomechanical properties (hard and damage tolerant) thanks to their hierarchical morphological structure throughout these levels. Nature’s approach is therefore ‘meso-scale’ (incorporating strategic nano-, micro- and macro-scale features), and it transforms brittle and weak elements into tough and resistant structures by assembling mineral component parts into a precise design and surrounding them with weak interfaces. The weakness of these interfaces is not as disadvantageous as may first appear, as they actually contribute to inducing a toughening mechanism.
Essentially, their flexibility acts as a shock absorber that thereby dissipates stress and multiplies the fatigue tolerance of the whole structure. The result of this strategy is particularly efficient, because it bestows isotropic characteristics on the overall complex, despite the highly anisotropic structures that may be observed on some hierarchical levels [80,81]. At the same time, the interactions among the different intrinsic optical properties, microstructure and histo-anatomic structures of the hard tissues create a very specific chromatic effect, which determines the overall esthetic appearance of the natural dentition. There are additional details about 3D printing that you can obtain in courses on our website.
3. The limitations of modern manufacturing of dental restorations
Computer-aided design and computer-aided manufacturing (CAD/CAM) technologies for the fabrication of dental restorations have rapidly spread worldwide [82]. To date, the most commonly used CAD/CAM methods in dentistry are subtractive: i.e., computer-controlled milling machines drill a block of material to achieve a desired morphology. Dentistry has profited greatly from this technology [83]. First, it makes it possible to create accurate restorations while significantly saving time [84]. Second, complex models can be built up more easily than with conventional methods [85]. Third, the use of monolithic blocks also means fewer internal defects, which are usually present in handmade restorations and which compromise their strength [86].
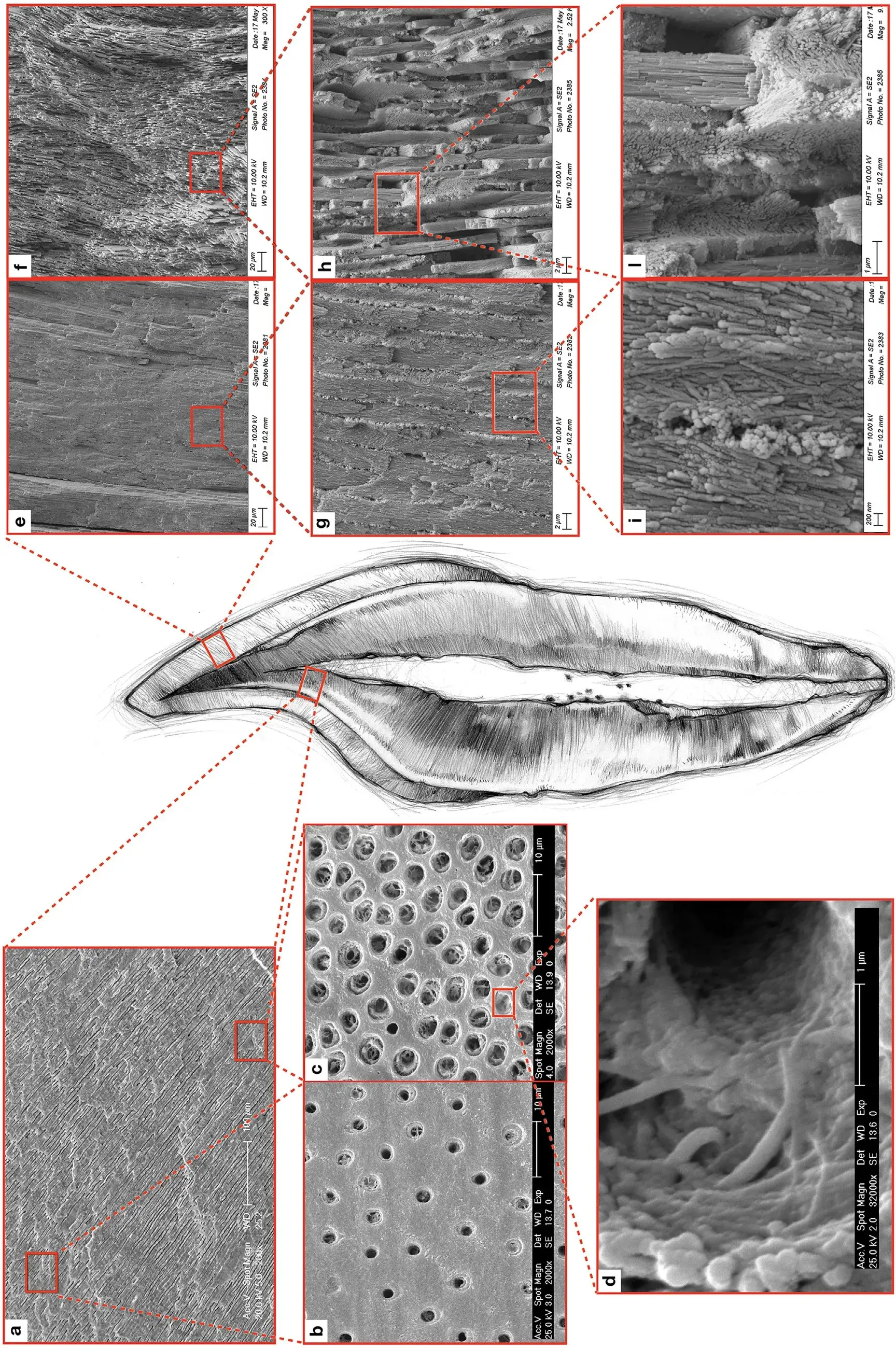 Fig. 1. The meso-scale structures – dentin (left), enamel (right) – of the tooth. The number, densities and dimensions of dentin tubuli are lower in the outer dentin (a) than in the inner dentin (b). Peritubular dentin presents a high degree of mineralization and a low organic rate, mostly represented by GAG, which forms the ‘lamina limitans’ completely embedded into the mineralized phase. On contrary, intertubular dentin shows a lower quantity of minerals and a higher organic rate, generally characterized by collagen type I (c). Each collagen fiber is composed of nano-fibrils (d). Rod arrangement varies from outer enamel (e and g), where rods are arranged in parallel, to inner enamel (f and h), where rods form a decussated pattern. Enamel rods are formed by bundle hydroxyapatite nano-crystals (i and l).
Fig. 1. The meso-scale structures – dentin (left), enamel (right) – of the tooth. The number, densities and dimensions of dentin tubuli are lower in the outer dentin (a) than in the inner dentin (b). Peritubular dentin presents a high degree of mineralization and a low organic rate, mostly represented by GAG, which forms the ‘lamina limitans’ completely embedded into the mineralized phase. On contrary, intertubular dentin shows a lower quantity of minerals and a higher organic rate, generally characterized by collagen type I (c). Each collagen fiber is composed of nano-fibrils (d). Rod arrangement varies from outer enamel (e and g), where rods are arranged in parallel, to inner enamel (f and h), where rods form a decussated pattern. Enamel rods are formed by bundle hydroxyapatite nano-crystals (i and l).
Table 2. A summary of materials, advantages and drawbacks of the most common technologies for additive manufacturing.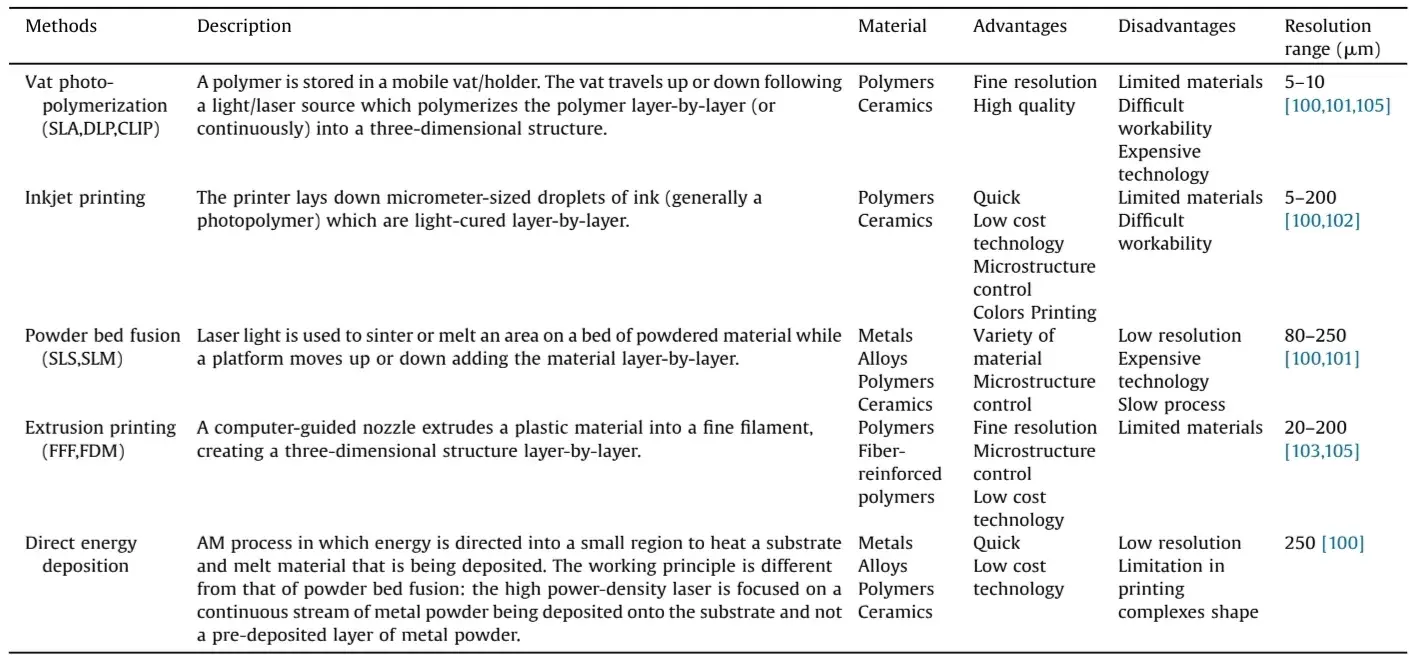
Despite all these advantages, subtractive processes are not free of limitations:
They waste raw material [82]. The material milled from blocks is difficult to recycle and is often considered as lost. Moreover, milling tools wear rapidly and thus need to be replaced frequently.
Milling procedures still require manual finishing, inevitably consuming more time. In fact, after manufacturing, the raw surfaces of restorations always need to be polished to remove the roughness left by the burs. A single small defect left by a bur may compromise the integrity of the entire restoration. This factor may be seen as critical when considering that milling processes may leave microscopic cracks on the surface of the material and thus rendering a restoration vulnerable [87,88].
The monolithic chromaticity of a milled restoration does not generally lend to outstanding esthetic results. Consequently, integrating a restoration from an esthetic point of view often involves color modification, either simply by painting the surface or by employing a more complex layering stratification. Those post-milling processes are not only time consuming but may also induce the integration of further defects during the manual retouching [89,90].
Modern CAD/CAM ceramic and resinous materials have high mechanical properties, far more than natural tissues and theoretically sufficient to withstand physiological stresses [91–93]. Yet they are less efficient than natural tissues in containing damage when it does occur, because their homogeneous internal microstructure does not replicate any of the above-mentioned toughening mechanisms found in natural tissues [94–96]. As a result, fracture remains one of the most common cause of clinical failure in all kinds of metal-free restorations [97].
In the light of these limitations and considerations, it seems opportune to reconsider the choice of computer-assisted manufacturing approach, and to think about future alternatives.
4. Future material for restorations: 3D-printing and topology optimization
Additive manufacturing (AM), or three-dimensional (3D)-printing, is a process that enables the creation of three-dimensional objects from digital data using the layer-by-layer deposition of material [98]. This method is already more than 30 years old [99]. The first working device, the stereolithography apparatus, was developed in 1984 by Hull, C. W. However, the mainstream adoption of 3D-printing only came about with the expiry of the last patents in 2009 and the subsequent fall in costs.
Since then, fast-paced development has boosted the accuracy, speed and reliability of 3D-printers, making them highly attractive for a wide range of fields. We can already see a broad variety of AM applications across several industries, including biomedicine and dentistry. Engineering improvements have led to the development of many different kinds of printing technology available nowadays, each with their own advantages and disadvantages (see Table 2) [100–105]. Each of these technologies is being increasingly applied to different medical areas, including dentistry.
In fact, 3D-printers are currently employed in many dental offices and laboratories to fabricate a wide array of occlusal splints [106], surgical guides [101–109], diagnostic models [110,111], orthodontic set-ups [112] and provisional restorations [113,114]. Moreover, 3D-printing seems to be a very promising technology which may pave the way for the development of new clinical strategies for bone regeneration surgery involving large bone defects [115–117].
Additive manufacturing has different advantages when compared to the subtractive approach. First, the waste material is reduced by around 40%, and the residue can be more readily recycled [118]. Second, the resolution of an additive process is much higher than with the subtractive approach. The precision of a 3D-printer depends on the kind of technology employed and on the thickness of the layers printable by the machine. However, modern 3D-printers are able to stratify layers in the range of 10–20mm, enabling the production of smooth surfaces and precise margins. This resolution is even high enough to eliminate the final stage of hand-polishing the surfaces of restorations, making the overall process potentially much faster [119]. Moreover, some preliminary in vitro tests have shown that restorations made using stereolithography (SLA) technology exhibited equivalent or higher precision marginal and internal adaptation than restorations made using milling [120,121].
Despite all these advantages, the use of printed objects in dentistry is still limited in many ways. The manufacturing of definitive restorations is not yet possible due to the lack of suitable materials. In subtractive manufacturing, the most commonly used materials for definitive restorations are reinforced ceramics, metals and composite resins. In additive manufacturing, some polymer prototypes and metals are starting to find their way into clinical practice, although 3D-printed ceramics for dental appli- cations are still struggling to make a name for themselves [122].
Metal is commonly used for conventional prosthodontic treatment. Laser-sintering 3D-printing techniques have been already used in the production of removable prosthesis frameworks with cobalt chromium [123]. Promising clinical outcome have also been reported with posterior single-unit metal–ceramic crowns [124,125]. However, despite its good mechanical properties, metal is not suited for modern micro-invasive and esthetic treatments. 3D-printed reinforced polymers show better potential for final restorations, but they will still need further development before being fit for purpose. In fact, the mechanical properties of actual resin-based 3D-printed workpieces are comparable to poly-methyl methacrylate (PMMA) commonly used for removable dentures and provisional restorations, which is far weaker than the highly filled resinous composites used in daily practice for bonded, direct or indirect definitive restorations. As a matter of fact, future research into the use of 3D-printing technologies in dentistry should focus on improving the mechanical properties of tooth-like materials, which could come about by developing new ‘bio-inspired’ microstructural arrangements inside a material. The most promising approach from today’s point of view would be to pursue topology optimization (TO). This mathematical method can be defined as a process to optimize the structural arrangement within the design of an object composed of one or more materials, with the goal of maximizing physical performance [126]. The approach was first introduced and described in 1994 by Sigmund, O. Initially, TO was used to focus on the modification of the macroscopic geometries of different objects designed using homogeneous materials [127]. With the development of multi-material 3D-printing techniques, it is now possible to work at a much higher resolution when combining different materials into designs that incorporate specific microstructures suited to a desired performance.
By breaking down the overall volume into the smallest essential digital volume units (voxels), TO optimizes their distribution to achieve a predefined functional objective [128,129]. This principle has already been tested in other fields, and the results are very encouraging. Drawing inspiration from natural materials Mirzaeifar et al., for example, [130] created samples composed of two distinct polymers with different mechanical properties set in a specific two-level hierarchical arrangement: stiff platelets inserted in a continuous soft phase. As a result, they demonstrated a significant improvement in the defect tolerance and physical properties of the new material and an outcome in terms of mechanics far superior to those of each of their constituents taken alone. Another research project improved by up to 200 times the toughness of the specimens by impregnating a soft resin into the micro-cracks of ceramic-like glass materials [131]. Also, another recent development in this field concerns fiber-reinforced composites, where micro-scale optimization has been used to customize the arrangement of fibers with a specific orientation. This new design allows fibers to achieve quasi-isotropic elastic properties, with a great benefit in terms of fracture toughness and damage tolerance [132].
For these reasons, future work might aim to mimic composition and meso-scale organization of natural tooth tissues to obtain similar toughening mechanisms. In other words, by working on the layout of micro-scale assemblies composed of one or more base materials, it would become possible to influence the macro-scale material properties to meet current needs in dental restorations.
The same approach could also be feasible in emulating the optical properties of the components of a tooth, in order to obtain more esthetic and natural results.
5. 3D bio-printing for regenerative dentistry
Regenerative tissue engineering aims to restore the function of injured tissue and organs through the production of cells and bioactive agents [133]. There is ample literature on the recreation of a range of biological tissue, including cardiac [134], hepatic [135], renal [136], skin [137] and bone tissue [138], all thanks to the recent development of 3D bio-printing technologies.
Extending these regeneration methods to tooth would offer new and innovative approaches to the widespread problem of edentulism. It has been reported that 40% of adults in Western countries present with one or more missing teeth, with this percentage increasing in developing countries [139,140]. The benchmark treatment today for a missing tooth is the titanium implant [141].
However, the 10-year survival rate of 82%–94% is not comparable to the 50-year survival rate of 99.5% for healthy teeth [142]. In the light of this, it appears appropriate to implement and develop the bio-manufacturing methods that are primarily used to regenerate healthy naturaltooth or atleast dentaltissues, such as endodontium and parodontium, thereby addressing irreversible pathologies.
Currently, 3D-printing technology is used to create scaffolds with precise architectures, with cells accurately embedded and following a design defined to recreate the biological structure of a specific tissue [143]. On the other hand, the printing of every component forming a tissue, includinglivingcells embedded in the extra-cellular matrices (ECM), is still in its early stages [144]. One obstacle is printing resolution, which is not yet high enough to recreate the complex nano-structure of tooth ECM, consisting of hydroxyapatite, collagen, and non-collagenous matrix proteins [145,146]. The biggest challenge for 3D bio-printing, however, is that the printing process must be cytocompatible and capable of reproducing in vitro a microenvironment that most closely represents the conditions of the tissue observed in vivo [147]. The ideal bio-ink for bio-printing wouldconsist of an aqueous gel solution containing naturalmolecules fromthe parenttissue [148],i.e.,the original ECM.
The ECM comprise a complex blend of proteins and other growth factors that provide mechanical, biophysical, and biochemical cues to cells in natural tissues. This mixture of components is essential not only to provide structural support but also to regulate resident cell differentiation [149], growth [150] and development [151].
Nowadays different bio-inks, based on a variety of hydrogels composed of de-cellularized ECM, are used to build scaffolds for different tissues. Recently, a bio-ink based on dentin-derived ECM, presenting high levels of cell survival at different concentrations and a high printability, has been successfully used [152].
Furthermore, 3D bio-printing has recently been used to regenerate periodontal tissue, where periodontal support has been lost, starting from hierarchical scaffolds capable of imitating the architectural organization of the periodontium, composed of hard (bone, cementum) and soft tissues (gingiva, periodontal ligament). The scaffolds are assembled in a multiphasic approach, as they are composed of various elements that recreate the original structure of the periodontal apparatus [153–155].
Lastly, it has been proposed that regenerating pulp tissue could be an alternative to conventional root canal treatment (RCT) [156,157]. Current RCT techniques typically involve the removal of infected and/or necrotic tissue and its replacement with inert synthetic biomaterials, thus sacrificing the biological response of the tooth [158]. Regenerative endodontics seeks instead to induce the re-vascularization and colonization of a new living pulp tissue, throughout the full-length of the root canal and pulp chamber, by the application of a mixture and an interpenetration of stem cells, bioactive molecules (e.g., growth factors) and 3D bio-printed scaffolds [159]. Although these new approaches appear highly promising, daily clinical employment is still far from becoming a reality [160].
6. Conclusion
The way in which teeth, nacre and other highly mineralized biological materials display such outstanding physical performance despite the weakness oftheir single constituent parts is a fascinating issue. An in-depth examination of the nano- and micro-architecture of these materials – including how they behave and how they fail – reveals ‘universal’ toughening rules, which should be reproduced in new ‘bio-inspired’ synthetic dental materials. Any such approach will be enabled by cutting-edge developments in additive manufacturing technologies which may open new pathways to enhance the properties of tooth-like substitute materials such as resinscomposite and ceramics.3D-printing technologiesalso appear very promising for the regeneration of dental tissue.
If you enjoyed reading this article and would like to explore the technologies in prosthetic dentistry further, we encourage you to enroll our course "Ceramic Veneers: the most demanded protocols in one place".
Acknowledgments
The authors thank Dr Adele Lodi Rizzini and Mr Edward Maxwell Crisp for the language proofreading, and Dr Tissiana Bortolotto for the imaging support.
List of authors:
Carlo Massimo Saratti, Giovanni Tommaso Rocca, Ivo Krejci
References
Lepora NF, Verschure P, Prescott TJ. The state of the art in biomimetics. Bioinspir Biomim 2013;8:013001.
Speck O, Speck D, Horn R, Gantner J, Sedlbauer KP. Biomimetic bio-inspired biomorph sustainable? An attempt to classify and clarify biology-derived technical developments. Bioinspir Biomim 2017;12:011004.
ISO 18458:2015 Biomimetics—terminology, concepts and methodology ISO 18458:2015-05 2015.
Gao H, Ji B, Jager IL, Arzt E, Fratzl P. Materials become insensitive to flaws at nanoscale: lessons from nature. Proc Natl Acad Sci U S A 2003;100:5597–600.
Fratzl P, Weinkamer R. Nature’s hierarchical materials. Prog Mater Sci 2007;52:1263–334.
Fratzl P. Biomimetic materials research: what can we really learn from nature’s structural materials? J R Soc Interface 2007;4:637–42.
Gebeshuber IC, Gruber P, Drack M. A gaze into the crystal ball: biomimetics in the year 2059. J Mech Eng Sci 2009;223:2899–918.
Munch E, Launey ME, Alsem DH, Saiz E, Tomsia AP, Ritchie RO. Tough, bioinspired hybrid materials. Science 2008;322:1516–20.
Luz GM, Mano JF. Biomimetic design of materials and biomaterials inspired by the structure of nacre. Philos Trans A Math Phys Eng Sci 2009;367:1587–605.
Wegst UG, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nat Mater 2015;14:23–36.
Ritchie RO. The conflicts between strength and toughness. Nat Mater 2011;10:817–22.
Khayer Dastjerdi A, Rabiei R, Barthelat F. The weak interfaces within tough natural composites: experiments on three types of nacre. J Mech Behav Biomed Mater 2013;19:50–60.
Chai H, Lee JJ, Constantino PJ, Lucas PW, Lawn BR. Remarkable resilience of teeth. Proc Natl Acad Sci U S A 2009;106:7289–93.
Braun S, Bantleon HP, Hnat WP, Freudenthaler JW, Marcotte MR, Johnson BE. A study of bite force, part 1: relationship to various physical characteristics. Angle Orthod 1995;65:367–72.
Nanci A. Ten cate’s oral histology: development, structure, and function. 8th ed. Elsevier Health Sciences; 2013.
Eanes ED. Enamel apatite: chemistry, structure and properties. J Dent Res 1979;58(Spec Issue B):829–36.
Gwinnett AJ. Structure and composition of enamel. Oper Dent 1992;suppl 5:10–7.
Koenigswald WV, Clemens WA. Levels of complexity in the microstructure of mammalian enamel and their application in studies of systematics. Scanning Microsc 1992;6:195–217.
Yilmaz ED, Schneider GA, Swain MV. Influence of structural hierarchy on the fracture behaviour of tooth enamel. Philos Trans A Math Phys Eng Sci 2015;373(2038).
Cui FZ, Ge J. New observations of the hierarchical structure of human enamel, from nanoscale to microscale. J Tissue Eng Regen Med 2007;1:185–91.
He LH, Swain MV. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J Mech Behav Biomed Mater 2008;1:18–29.
Sognnaes RF. The organic elements of the enamel; the gross morphology and the histological relationship of the lamellae to the organic framework of the enamel. J Dent Res 1950;29:260–9.
Osborn JW. The 3-dimensional morphology of the tufts in human enamel. Acta Anat (Bosel) 1969;73:481–95.
Bechtle S, Özcoban H, Lilleodden ET, Huber N, Schreyer A, Swain MV, et al. Hierarchical flexural strength of enamel: transition from brittle to damage-tolerant behaviour. J R Soc Interface 2012;9:1265–74.
Imbeni V, Kruzic JJ, Marshall GW, Marshall SJ, Ritchie RO. The dentin-enamel junction and the fracture of human teeth. Nat Mater 2005;4:229–32.
Marshall SJ, Balooch M, Habelitz S, Balooch G, Gallagher R, Marshall GW. The dentin–enamel junction—a natural, multilevel interface. J Eur Ceramic Soc 2003;23:1897–904.
Staines M, Robinson WH, Hood JAA. Spherical indentation of tooth enamel. J Mater Sci 1981;16:2551–6.
Hassan R, Caputo AA, Bunshah RF. Fracture toughness of human enamel. J Dent Res 1981;60:820–7.
Willems G, Celis JP, Lambrechts P, Braem M, Vanherle G. Hardness Young’s modulus determined by nanoindentation technique of filler particles of dental restorative materials compared with human enamel. J Biomed Mater Res 1993;27:747–55.
Lin CP, Douglas WH. Structure-property relations and crack resistance at the bovine dentin-enamel junction. J Dent Res 1994;73:1072–8.
Meredith N, Sherriff M, Setchell DJ, Swanson SA. Measurement of the microhardness and Young’s modulus of human enamel and dentine using an indentation technique. Arch Oral Biol 1996;41:539–45.
Xu HH, Smith DT, Jahanmir S, Romberg E, Kelly JR, Thompson VP, et al. Indentation damage and mechanical properties of human enamel and dentin. J Dent Res 1998;77:472–80.
Habelitz S, Marshall SJ, Marshall Jr. GW, Balooch M. Mechanical properties of human dental enamel on the nanometre scale. Arch Oral Biol 2001;46:173–83.
Marshall Jr. GW, Balooch M, Gallagher RR, Gansky SA, Marshall SJ. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J Biomed Mater Res 2001;54:87–95.
White SN, Luo W, Paine ML, Fong H, Sarikaya M, Snead ML. Biological organization of hydroxyapatite crystallites into a fibrous continuum toughens and controls anisotropy in human enamel. J Dent Res 2001;80:321–6.
Balooch G, Marshall GW, Marshall SJ, Warren OL, Asif SA, Balooch M. Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J Biomech 2004;37:1223–32.
Rizkalla AS, Jones DW. Indentation fracture toughness and dynamic elastic moduli for commercial feldspathic dental porcelain materials. Dent Mater 2004;20:198–206.
Mann AB, Dickinson ME. Nanomechanics, chemistry and structure at the enamel surface. Monogr Oral Sci 2006;19:105–31.
Sakar-Deliormanli A, Güden M. Microhardness and fracture toughness of dental materials by indentation method. J Biomed Mater Res B Appl Biomater 2006;76:257–64.
Park S, Quinn JB, Romberg E, Arola D. On the brittleness of enamel and selected dental materials. Dent Mater 2008;24:1477–85.
Thomas HF, Carella P. A scanning electron microscope study of dentinal tubules from un-erupted human teeth. Arch Oral Biol 1983;28:1125–30.
Bertassoni LE, Stankoska K, Swain MV. Insights into the structure and composition of the peritubular dentin organic matrix and the lamina limitans. Micron 2012;43:229–36.
Symons NB. Interglobular dentin and the calcospherite pattern. Arch Oral Biol 1965;10:1009–10.
Thomas HF. The lamina limitans of human dentinal tubules. J Dent Res 1984;63:1064–6.
Bertassoni LE. Dentin on the nanoscale: hierarchical organization, mechanical behavior and bioinspired engineering. Dent Mater 2017;33:637–49.
Ho SP, Sulyanto RM, Marshall SJ, Marshall GW. The cementum-dentin junction also contains glycosaminoglycans and collagen fibrils. J Struct Biol 2005;151:69–78.
Bertassoni LE, Kury M, Rathsam C, Little CB, Swain MV. The role of proteoglycans in the nanoindentation creep behavior of human dentin. J Mech Behav Biomed Mater 2015;55:264–70.
Bertassoni LE, Swain MV. Removal of dentin non-collagenous structures results in the unraveling of microfibril bundles in collagen type I. Connect Tissue Res 2017;58:414–23.
Lawn BR, Bush MB, Barani A, Constantino P, Wroe S. Inferring biological evolution from fracture patterns in teeth. J Theor Biol 2013;338:59–65.
Barani A, Chai H, Lawn BR, Bush MB. Mechanics analysis of molar tooth splitting. Acta Biomater 2015;15:237–43.
Lee JJ, Kwon JY, Chai H, Lucas PW, Thompson VP, Lawn BR. Fracture modes in human teeth. J Dent Res 2009;88:224–8.
Chai H, Lee JJ, Kwon JY, Lucas PW, Lawn BR. A simple model for enamel fracture from margin cracks. Acta Biomater 2009;5:1663–7.
Barani A, Keown AJ, Bush MB, Lee JJ, Chai H, Lawn BR. Mechanics of longitudinal cracks in tooth enamel. Acta Biomater 2011;7:2285–92.
Yahyazadehfar M, Bajaj D, Arola DD. Hidden contributions of the enamel rods on the fracture resistance of human teeth. Acta Biomater 2013;9:4806–14.
Barani A, Bush MB, Lawn BR. Effect of property gradients on enamel fracture in human molar teeth. J Mech Behav Biomed Mater 2012;15:121–30.
Bechtle S, Habelitz S, Klocke A, Fett T, Schneider GA. The fracture behaviour of dental enamel. Biomaterials 2010;31:375–84.
Yilmaz ED, Schneider GA. Mechanical behaviour of enamel rods under micro-compression. J Mech Behav Biomed Mater 2016;63:183–94.
Özcoban H, Yilmaz ED, Schneider GA. Hierarchical microcrack model for materials exemplified at enamel. Dent Mater 2018;34:69–77.
Bechtle S, Fett T, Rizzi G, Habelitz S, Klocke A, Schneider GA. Crack arrest within teeth at the dentinoenamel junction caused by elastic modulus mismatch. Biomaterials 2010;31:4238–47.
Dong XD, Ruse ND. Fatigue crack propagation path across the dentinoenamel junction complex in human teeth. J Biomed Mater Res A 2003;66:103–9.
Chai H, Lee JJ-W, Lawn BR. On the chipping and splitting of teeth. J Mech Behav Biomed Mater 2011;4:315–21.
Ivancik J, Arola DD. The importance of microstructural variations on the fracture toughness of human dentin. Biomaterials 2013;34:864–74.
Bertassoni LE, Swain MV. The contribution of proteoglycans to the mechanical behavior of mineralized tissues. J Mech Behav Biomed Mater 2014;38:91–104.
Arola DD, Reprogel RK. Tubule orientation and the fatigue strength of human dentin. Biomaterials 2006;22:2131–40.
Montoya C, Arola D, Ossa EA. Importance of tubule density to the fracture toughness of dentin. Arch Oral Biol 2016;67:9–14.
Yahyazadehfar M, Zhang D, Arola D. On the importance of aging to the crack growth resistance of human enamel. Acta Biomater 2016;32:264–74.
Arola DD, Rouland JA. The effects of tubule orientation on fatigue crack growth in dentin. J Biomed Mater Res A 2003;67:78–86.
Bazos P, Magne P. Bio-emulation: biomimetically emulating nature utilizing a histo-anatomic approach; structural analysis. Eur J Esthet Dent 2011;6:8–19.
Jahangiri L, Reinhardt SB, Mehra RV, Matheson PB. Relationship between tooth shade value and skin color: an observational study. J Prosthet Dent 2002;87:149–52.
Ten Bosch JJ, Zijp JR. Optical properties of dentin. In: Thylstrup A, Leach SA, Qvist V, editors. Dentine and dentine reactions in the oral cavity. Oxford, England: IRL Press; 1987. p. 59–65.
Zijp JR, Ten Bosch JJ. Theoretical model for scattering of light by dentin and comparison with measurements. Appl Opt 1993;32:411–5.
Zijp JR, Ten Bosch JJ, Groenhuis RAJ. HeNe-laser scattering by human dental enamel. J Dent Res 1995;74:1891–8.
Vaarkamp J, Ten Bosch JJ, Verdonschot EH. Propagation of light through human dental enamel and dentine. Caries Res 1995;29:8–13.
Winter R. Visualizing the natural dentition. J Esthet Dent 1993;5:102–17.
Ten Bosch JJ, Coops JC. Tooth color and reflectance as related to light scattering and enamel hardness. J Dent Res 1995;74:374–80.
JoinerA. Toothcolour: a review ofthe literature.J Dent 2004;32(Suppl 1):3–12.
Goodkind RJ, Schwabacher WB. Use of a fiber-optic colorimeter for an in vivo color measurement of 2830 anterior teeth. J Prosthet Dent 1987;58:535–42.
Zhao Y, Zhu J. In vivo color measurement of 410 maxillary anterior teeth. Chin J Dent Res 1998;1:49–51.
Russell MD, Gulfraz M, Moss BW. In vivo measurement of colour changes in natural teeth. J Oral Rehabil 2000;27:786–92.
Weiner S, Addadi L, Wagner HD. Materials design in biology. Mater Sci Eng C 2000;11:1–8.
Luz GM, Mano JF. Mineralized structures in nature: examples and inspirations for the design of new composite materials and biomaterials. J Comp Sci Technol 2010;70:1777–88.
Van Noort R. The future of dental devices is digital. Dent Mater 2012;28:3–12.
Alghazzawi TF. Advancements in CAD/CAM technology: options for practical implementation. J Prosthodont Res 2016;60:72–84.
Bindl A, Mörmann WH. Marginal and internal fit of all-ceramic CAD/CAM crown-copings on chamfer preparations. J Oral Rehabil 2005;32:441–7.
Yuzbasioglu E, Kurt H, Turunc R, Bilir H. Comparison of digital and conventional impression techniques: evaluation of patients’ perception, treatment comfort, effectiveness and clinical outcomes. BMC Oral Health 2014;30:10.
Belli R, Wendler M, de Ligny D, Cicconi MR, Petschelt A, Peterlik H, et al. Chairside CAD/CAM materials. Part 1: measurement of elastic constants and microstructural characterization. Dent Mater 2017;33:84–98.
Canneto JJ, Cattani-Lorente M, Durual S, Wiskott AH, Scherrer SS. Grinding damage assessment on four high-strength ceramics. Dent Mater 2016;32:171–82.
Curran P, Cattani-Lorente A, Wiskott AHW, Durual S, Scherrer SS. Grinding damage assessment for CAD-CAM restorative materials. Dent Mater 2017;33:294–308.
Fischer J, Stawarzcyk B, Trottmann A, Hammerle CH. Impact of thermal misfit on shear strength of veneering ceramic/zirconia composites. Dent Mater 2009;25:419–23.
Gostemeyer G, Jendras M, Dittmer MP, Bach F, Stiesch M, Kohorst P. Influence of cooling rate on zirconia/veneer interfacial adhesion. Acta Biomater 2010;6:4532–8.
Li RW, Chow TW, Matinlinna JP. Ceramic dental biomaterials and CAD/CAM technology: state of the art. J Prosthodont Res 2014;58:208–16.
Wendler M, BelliR, PetscheltA, Mevec D, HarrerW, Lube T, et al. Chairside CAD/CAM materials. Part 2: flexural strength testing. Dent Mater 2017;33:99–109.
Lohbauer U, Reich S. Antagonist wear of monolithic zirconia crowns after 2 years. Clin Oral Investig 2017;21:1165–72.
Belli R, Petschelt A, Lohbauer U. Are linear elastic material properties relevant predictors of the cyclic fatigue resistance of dental resin composites. Dent Mater 2014;30:381–91.
Belli R, Geinzer E, Muschweck A, Petschelt A, Lohbauer U. Mechanical fatigue degradation of ceramics versus resin composites for dental restorations. Dent Mater 2014;30:424–32.
Belli R, Wendler M, Zorzin JI, Lohbauer U. Practical and theoretical considerations on the fracture toughness testing of dental restorative materials. Dent Mater 2018;34:97–119.
Belli R, Petschelt A, Hofner B, Hajtó J, Scherrer SS, Lohbauer U. Fracture rates and lifetime estimations of CAD/CAM All-ceramic restorations. J Dent Res 2016;95:67–73.
Berman B. 3-D printing: the new industrial revolution. Bus Horizons 2012;55:155–62.
Attaran M. The rise of 3-D printing: the advantages of additive manufacturing over traditional manufacturing. Bus Horizons 2017;60:677–88.
NgoTD, Kashani A, Imbalzano G, Nguyen KTQ, Hui D. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Composites B Eng 2018;143:172–96.
Wang X, Jiang M, Zhou Z, Gou J, Hui D. 3D printing of polymer matrix composites: a review and prospective. Composites B Eng 2017;110: 442–58.
Kazemian A, Yuan X, Cochran E, Khoshnevis B. Cementitious materials for construction-scale 3D printing: laboratory testing of fresh printing mixture. Constr Build Mat 2017;145:639–47.
Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J Dent Res 2015;94:143S–52S.
Dawood A, Marti B, Sauret-Jackson V, Darwood A. 3D printing in dentistry. Br Dent J 2015;219:521–9.
Stansbury JW, Idacavage MJ. 3D printing with polymers: challenges among expanding options and opportunities. Dent Mater 2016;32:54–64.
Salmi M, Paloheimo KS, Tuomi J, Ingman T, Mäkitie A. A digital process for additive manufacturing of occlusal splints: a clinical pilot study. J R Soc Interface 2013;10: 20130203.
Di Giacomo GA, da Silva JV, da Silva AM, Paschoal GH, Cury PR, Szarf G. Accuracy and complications of computer-designed selective laser sintering surgical guides for flapless dental implant placement and immediate definitive prosthesis installation. J Periodontol 2012;83:410–9.
Di Giacomo GA, Cury PR, da Silva AM, da Silva JV, Ajzen SA. A selective laser sintering prototype guide used to fabricate immediate interim fixed complete arch prostheses in flapless dental implant surgery: technique description and clinical results. J Prosthet Dent 2016;116:874–9.
Giacomo GD, Silva J, Martines R, Ajzen S. Computer-designed selective laser sintering surgical guide and immediate loading dental implants with definitive prosthesis in edentulous patient: a preliminary method. Eur J Dent 2014;8:100–6.
Salmi M. Possibilities of preoperative medical models made by 3D printing or additive manufacturing. J Med Eng 2016;2016: 6191526.
Bukhari S, Goodacre BJ, AlHelal A, Kattadiyil MT, Richardson PM. Three-dimensional printing in contemporary fixed prosthodontics: a technique article. J Prosthet Dent 2018;119:530–4.
Groth C, Kravitz ND, Shirck JM. Incorporating three-dimensional printing in orthodontics. J Clin Orthod 2018;52:28–33.
Joo HS, Park SW, Yun KD, Lim HP. Complete-mouth rehabilitation using a 3D printing technique and the CAD/CAM double scanning method: a clinical report. J Prosthet Dent 2016;116:3–7.
Tahayeri A, Morgan M, Fugolin AP, Bompolaki D, Athirasala A, Pfeifer CS, et al.
3D printed versus conventionally cured provisional crown and bridge dental materials. Dent Mater 2018;34:192–200.
Carrel JP, Wiskott A, Moussa M, Rieder P, Scherrer S, Durual S. A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin Oral Implants Res 2016;27:55–62.
Carrel JP, Wiskott A, Scherrer S, Durual S. Large bone vertical augmentation using a three-dimensional printed TCP/HA bone graft: a pilot study in dog mandible. Clin Implant Dent Relat Res 2016;18:1183–92.
Fahimipour F, Dashtimoghadam E, Rasoulianboroujeni M, Yazdimamaghani M, Khoshroo K, Tahriri M, et al. Collagenous matrix supported by a 3D-printed scaffold for osteogenic differentiation of dental pulp cells. Dent Mater 2018;34:209–20.
Petrovic V, Haro Gonzalez JV, Ferrando OJ, Gordillo JD, Blanco Puchades JR, Griñan LP. Additive layered manufacturing: sectors of industrial application shown through case studies. Int J Prod Res 2011;49:1071–9.
Schweiger J, Beuer F, Stimmelmayr M, Edelhoff D, Magne P, Güth JF. Histoanatomic 3D printing of dental structures. Br Dent J 2016;221:555–60.
Mai HN, Lee KB, Lee DH. Fit of interim crowns fabricated using photopolymer-jetting 3D printing. J Prosthet Dent 2017;118:208–15.
Alharbi N, Alharbi S, Cuijpers VMJI, Osman RB, Wismeijer D. Three- dimensional evaluation of marginal and internal fit of 3D-printed interim restorations fabricated on different finish line designs. J Prosthodont Res 2018;62:218–26.
Dehurtevent M, Robberecht L, Hornez JC, Thuault A, Deveaux E, Béhin P. Stereolithography: a new method for processing dental ceramics by additive computer-aided manufacturing. Dent Mater 2017;33:477–85.
Laverty DP, Thomas MBM, Clark P, Addy LD. The use of 3D metal printing (direct metal laser sintering) in removable prosthodontics. Dent Update 2016;43: 826–8, 831–2, 834–5.
Tara MA, Eschbach S, Bohlsen F, Kern M. Clinical outcome of metal–ceramic crowns fabricated with laser-sintering technology. Int J Prosthodont 2011;24:46–8.
Atzeni E, Salmi A. Evaluation of additive manufacturing (AM) techniques for the production of metal–ceramic dental restorations. J Manuf Processesg 2015;20:40–5.
Bendsøe MP, Sigmund O. Topology optimization: theory, methods and applications. Springer Science & Business Media; 2004.
Sigmund O. Materials with prescribed constitutive parameters: an inverse homogenization problem. Int J Sol Struct 1994;31:2313–29.
Zhu B, Skouras M, Chen D, Matusik W. Two-scale topology optimization with microstructures. ACM Trans Graph 2017;34:84.
Velasco-Hogan A, Xu J, Meyers MA. Additive manufacturing as a method to design and optimize bioinspired structures. Adv Mater 1800;2018:940.
Mirzaeifar R, Dimas LS, Qin Z, Buehler MJ. Defect-tolerant bioinspired hierarchical composites: simulation and experiment. ACS Biomater Sci Eng 2015;1:295–304.
Mirkhalaf M, Dastjerdi AK, Barthelat F. Overcoming the brittleness of glass through bio-inspiration and micro-architecture. Nat Commun 2014;5:3166.
Raney JR, Comptona BG, Mueller J, Obera TJ, Shead K, Lewis JA. Rotational 3D printing of damage-tolerant composites with programmable mechanics. Proc Natl Acad Sci U S A 2018;115:1198–203.
Koledova Z. 3D cell culture: methods and protocols, methods in molecular biology, vol. 1612. Springer Science & Business Media LLC; 2017.
Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, et al. Bioprinting 3D microfibroun scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016;110:45–59.
Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo WA, et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 2014;6: 024105.
Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 2016;6: 34845.
Cubo N, Garcia M, Del Cañizo JF, Velasco D, Jorcano JL. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 2016;9: 015006.
Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 2016;34:312–9.
Garcia-Godoy F, Murray PE. Status and potential commercial impact of stem cell-based treatments on dental and craniofacial regeneration. Stem Cells Dev 2006;15:881–7.
Hacking SA, Khademhosseini A. Applications of microscale technologies for regenerative dentistry. J Dent Res 2009;88:409–21.
Ali Z, Baker SR, Shahrbaf S, Martin N, Vettore MV. Oral health-related quality of life after prosthodontic treatment for patients with partial edentulism: a systematic review and meta-analysis. J Prosthet Dent 2018 pii: S0022-3913 (18)30229-4.
Holm-Pedersen P, Lang NP, Müller F. What are the longevities of teeth and oral implants. Clin Oral Implants Res 2007;18:15–9.
Derby B. Printing and prototyping of tissues and scaffolds. Science 2012;338:921–6.
Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J Dent Res 2015;94(9 Suppl):143S–52S.
Kim BS, Kim H, Gao G, Jang J, Cho DW. Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication 2017;9: 034104.
Choudhury D, Tun HW, Wang T, Naing MW. Organ-derived decellularized extracellular matrix: a game changer for bioink manufacturing? Trends Biotechnol 2018;36:787–805.
Ferris CJ, Gilmore KG, Wallace GG, In het Panhuis M. Biofabrication: an overview of the approaches used for printing of living cells. Appl Microbiol Biotechnol 2013;97:4243–58.
Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 2014;5:3935.
Chastain SR, Kundu AK, Dhar S, Dhar JW, Putnam AJ. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J Biomed Mater Res A 2006;78:73–85.
Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res 2008;68:3185–92.
Simon-Assmani P, Kedinger M, De Arcangelis A, Rousseau V, Simo P. Extracellular matrix components in intestinal development. Experientia 1995;51:883–900.
Monteiro N, Thrivikraman G, Athirasala A, Tahayeri A, França CM, Ferracane JL, et al. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent Mater 2018;34:389–99.
Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, Bartold PM. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res 2014;93:1212–21.
Vaquette C, Fan W, Xiao Y, Hamlet S, Hutmacher DW, Ivanovski S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012;33:5560–73.
Costa PF, Vaquette C, Zhang Q, Reis RL, Ivanovski S, Hutmacher DW. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J Clin Periodontol 2014;41:283–94.
Saghiri MA, Asatourian A, Sorenson CM, Sheibani N. Role of angiogenesis in endodontics: contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J Endod 2015;41: 797–803.
Athirasala A, Lins F, Tahayeri A, Hinds M, Smith AJ, Sedgley C, et al. A novel strategy to engineer pre-vascularized full-length dental pulp-like tissue constructs. Sci Rep 2017;7:3323.
Smith AJ, Lumley PJ, Tomson PL, Cooper PR. Dental regeneration and materials: a partnership. Clin Oral Investig 2008;12:103–8.
Albuquerque MT, Valera MC, Nakashima M, Nör JE, Bottino MC. Tissueengineering-based strategies for regenerative endodontics. J Dent Res 2014;93:1222–31.
Athirasala A, Tahayeri A, Thrivikraman G, França CM, Monteiro N, Tran V, et al. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018;10: 024101.


