Surface electromyography of jaw muscles and kinesiographic recordings: diagnostic accuracy for myofascial pain
SUMMARY
The present investigations attempted to assess the diagnostic accuracy of commercially available surface electromyography (sEMG) and kinesiography (KG) devices for myofascial pain of jaw muscles. Thirty-six (n = 36) consecutive patients with a research diagnostic criteria for temporomandibular disorders (RDC ⁄TMD) axis I diagnosis of myofascial pain and an age- and sex-matched group of 36 TMD-free asymptomatic subjects underwent sEMG and KG assessments to compare EMG parameters of the masseter and temporalis muscles as well as the jaw range of motion and the interarch freeway space. EMG data at rest were not significantly different between myofascial pain patients and asymptomatic subjects, while the latter achieved significantly higher levels of EMG activity during clenching tasks. Symmetry of muscle activity at rest and during clenching tasks, KG parameters of jaw range of motion and the measurement of the interarch vertical freeway did not differ between groups. Receiver operating characteristics curve analysis showed that, except EMG parameters during clenching tasks, all the other outcome sEMG and KG measures did not reach acceptable levels of sensitivity and specificity, with a 30Æ6–88Æ9% percentage of false-positive results. Therefore, clinicians should not use sEMG and KG devices as diagnostic tools for individual patients who might have myofascial pain in the jaw muscles. Whether intended as a stand-alone measurement or as an adjunct to making clinical decisions, such instruments do not meet the standard of reliability and validity required for such usage.
You have the opportunity to gather more in-depth information about temporomandibular disorders in our Online congress of evidence-based temporomandibular disorders and bruxism treatment.
INTRODUCTION
The issue of temporomandibular disorders (TMD) diagnosis is one of the most controversial fields in dentistry (1). Clinical diagnosis performed by means of an accurate history taking and standardised examinations is considered the standard of reference (2), and supplemental information via imaging techniques may be useful in some selected cases (3). The role of bioelectronic devices has been diminished after some systematic reviews suggested that they can provide, at best, no more than ancillary documentation (4–7). Notwithstanding that, claims for the usefulness of technological devices in the daily TMD practice are still diffused among clinical practitioners (8, 9). Also, it seems that the quality of literature on the use of devices such as surface electromyography (sEMG) and kinesiography (KG) is poor, and the lack of normative values on which the discriminatory power between TMD patients and asymptomatic subjects should be based is a strong limitation for a definitive appraisal of their validity. Besides, it is unclear whether such instruments are claimed to detect specific subsets of TMD patients.
Thus, an improvement in the quality of investigations on this issue is a compelling need to be achieved through appropriately designed case–control studies. In view of these considerations, the present investigations attempted to assess the diagnostic ACCURACY of commercially available sEMG and KG devices for myofascial pain of jaw muscles, which is supposed to be the main target for instruments aiming at detecting the muscle activity and the patterns of jaw motion for diagnostic purposes.
MATERIALS AND METHODS
A priori sample size calculation
A priori calculation of the needed sample size to detect clinically significant differences between a group of patients with myofascial pain of jaw muscles and a group of asymptomatics was based on data drawn from the literature, taking resting sEMG values as the main outcome parameter.
A 50% difference with respect to 2Æ5 lV, which was suggested to be the cut-off for abnormal sEMG values (8), was set as the difference to detect. Expected variance was set at 3 lV on the basis of an estimated standard deviation comprised between 1Æ5 and 2Æ5 lV. This meant that a sample size of about 30 subjects per group was needed to achieve an 80% statistical power (b error set at 0Æ20) to detect a clinically significant difference with a 5% probability to have a false-positive error (a error set at 0Æ05).
Study sample and design
A group of 36 consecutive patients (24 women, 12 men; mean age 34 - 9 years) seeking for TMD treatment at the TMD Clinic, Department of Maxillofacial Surgery, University of Padova, Italy, and receiving a research diagnostic criteria for temporomandibular disorders(RDC ⁄ TMD) axis I diagnosis of myofascial pain (10) either without (Ia; n = 25) or with limited opening (Ib; n = 11) underwent a sEMG and KG assessments in accordance with the protocol described later. An age- and sex-matched group of 36 TMD-free subjectswith no RDC ⁄ TMD axis I diagnoses were recruited among the university staff and their closest friends. The latter control group was strictly composed by subjects who did not enter in any contact in the past with either the researchers involved in the investigations or the instruments under investigation to avoid potential bias because of preconceived ideas. The presence of concurrent multiple diagnoses along with myofascial pain, viz. joint disorders, in the study group was allowed. All RDC ⁄ TMD assessments were made by one of two trained examiners (D.M; L.G.N.) already involved in other RDC ⁄ TMD researches (11, 12). All myofascial pain patients presented pain at the time of the EMG recording session with an average VAS level of six points on a 0–10 rating scale.
Surface electromyography and kinesiographic recordings
All study participants underwent an electromyographic and kinesiographic recording with a commercially available device*. During all examinations, the patient was sat on a wooden high-backed chair, with the trunk perpendicular to the floor and the head upright.
According to the manufacturer’s protocol, the sEMG assessment was recorded by the use of bipolar surface electrodes†, bilaterally placed on the subject’s skin overlying the body of masseter muscle and the anterior temporalis muscle. After the EMG activity at rest was recorded, the patient was asked to clench the teeth maximally three times for 2 s, with 2-s relaxation between each clench. The clenching exercise was repeated also with a cotton roll positioned over the posterior teeth of both sides, as suggested by the manufacturer’s guidelines. The kinesiographic recordings were made with the use of a magnet temporarily applied on the subject’s buccal mucosa under the lower arch central incisors to monitor the location of the mandible against a sensor array suspended in front of the face by a lightweight frame suspended on the bridge of the nose and connected behind the head by straps. All tasks were performed three times at ten-minute intervals, and the average value of the three attempts was recorded. All sEMG and KG assessments were made by one of two investigators (S.T.; F.C.) with expertise in the use of such devices and with continued education training at in-house courses organised by the manufacturer. The examiners were blinded to the participants’ status, viz. being a patient or a control.
For all participants, the following parameters were recorded and considered as outcome variables for group comparison: maximum mouth opening (in mm), maximum lateral deviations from the mid-sagittal plane during jaw opening (in mm); vertical free-way space (in mm); resting EMG values for the four investigated muscles (in lV); EMG values during maximum clenching on tooth and on cotton rolls (in lV); and symmetry of muscle function, assessed as a raw ratio between the right and left muscle activity for masseter and temporalis muscles.
Statistical analysis
The average values in jaw range of motion and EMG activity were managed as continuous variables, and the existence of between-group differences was tested by the adoption of a t-test for independent samples. The level for statistical significance was set at P < 0Æ05.
A receiver operating characteristics (ROC) curve analysis was performed to detect diagnostic accuracy (area under the curve), true-positive rate [TPR (sensitivity)] and false-positive rate [FPR (1-specificity)] of each parameter to discriminate between patients and controls. Receiver operating characteristics curve analysis interpretation was based on the assumption that an area of 1 represents a perfect test, while an area of 0Æ5 represents a worthless test, viz. not superior to a coin toss. The closer the curve follows the left-hand border and then the top border of the ROC space, the more accurate the test; the TPR is high and the FPR is low. Statistically, a larger area under the curve means that it is identifying more true positives while minimising the percentage of false positives (13). All statistical procedures were performed with a dedicated software.
RESULTS
Sex differences were not significant for any of the outcome measures (Table 1). Ranges of EMG data at rest in the four investigated muscles were WITH IN the 2Æ2–4Æ0 lV range in TMD patients and within the 2Æ9–3Æ8 lV range in TMD-free subjects. Differences between the two groups were not significant, except for the left masseter muscles, which showed a higher resting EMG activity in the control group (Table 2). EMG activity markedly increased during clenching tasks in both groups, and TMD-free subjects achieved significantly higher levels of EMG activity for all the FOUR investigated muscles. EMG activity during clenching on natural teeth was 64Æ1–79Æ2 lV in TMD patients and 140Æ2–182Æ8 lV in control subjects, while the corresponding values for clenching on cotton rolls were 70Æ1–78Æ7 and 151Æ3–217Æ3 lV, respectively (Table 3).
Measures of jaw range of motion and patterns of movements were similar in the two groups, except a higher deflection to the right during jaw opening in the control group (2Æ7 versus 1Æ7 mm). Also, the interarch freeway space in rest position did not differ between TMD patients (1Æ7 mm) and TMD-free subjects (1Æ4 mm) (Table 4). No between-group differences were detected as for the ratio between right and left muscle activity in patients and controls.
Receiver operating characteristics curve analysis showed that fair to excellent accuracy (>0Æ7) to discriminate between the two groups was achieved only with EMG parameters during clenching tasks (Figs 1–4). Clenching tasks also showed acceptable levels of sensitivity (true-positive rate, 77Æ8–91Æ7%) and specificity (76Æ7–86Æ7%). Resting EMG values had unacceptable levels of accuracy (0Æ28–0Æ48), sensitivity (43Æ5–52Æ2) and specificity (27Æ8–55Æ6). Also, kinesiographic recordings of jaw movement patterns, measures of interarch freeway space and the ratio of symmetric muscle activity did not reach acceptable levels of sensitivity and specificity (Table 5).
Table 1. Sex differences in the study parameters
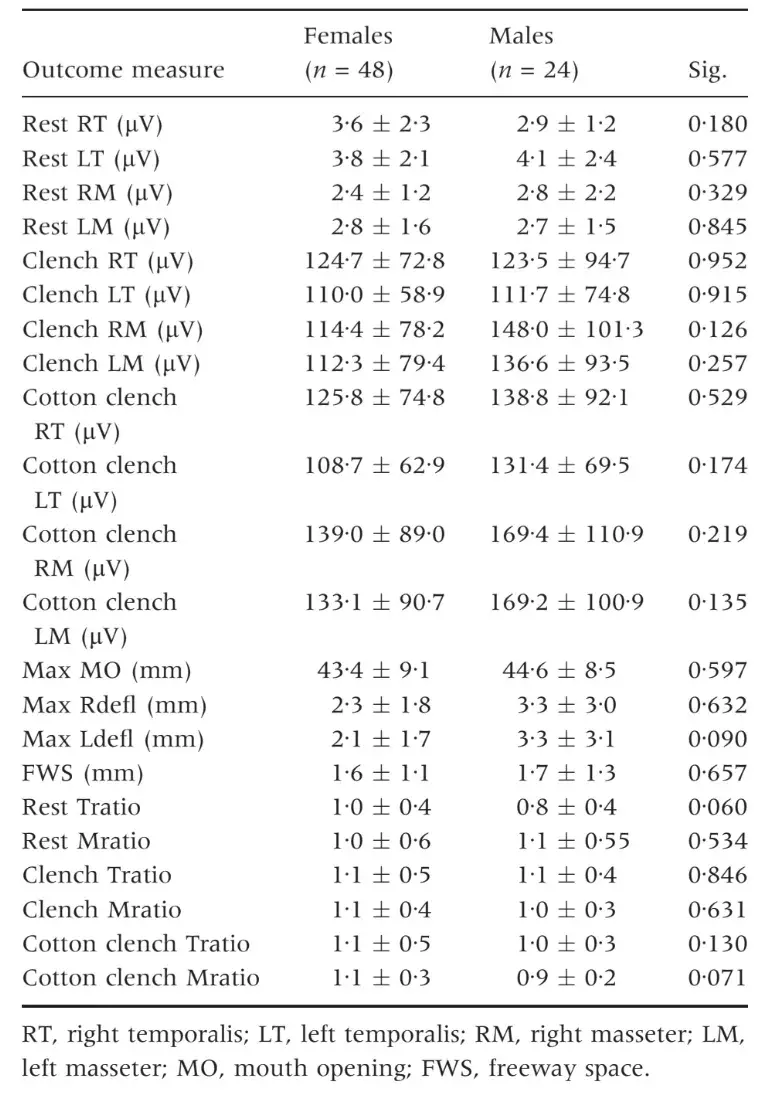
Table 2. Electromyography values at rest. Comparison between patients and controls
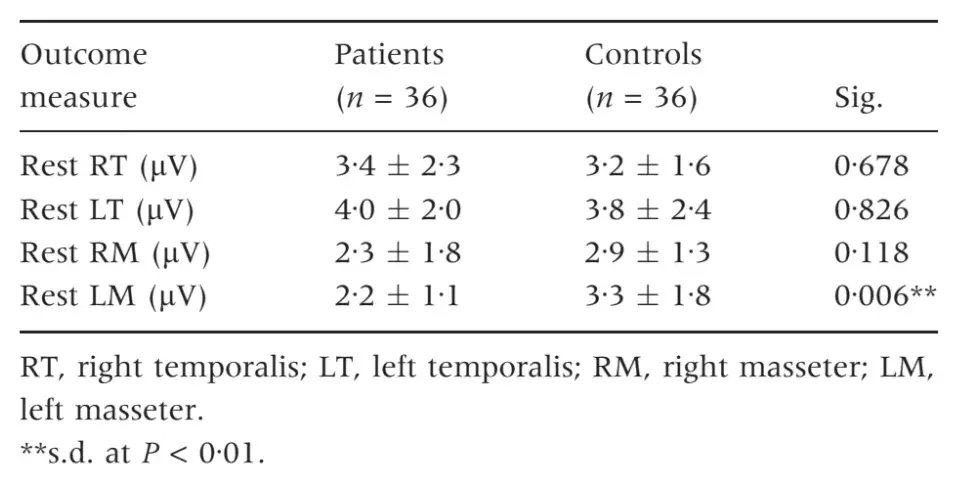
Table 3. Electromyography values during clenching tasks. Comparison between patients and controls
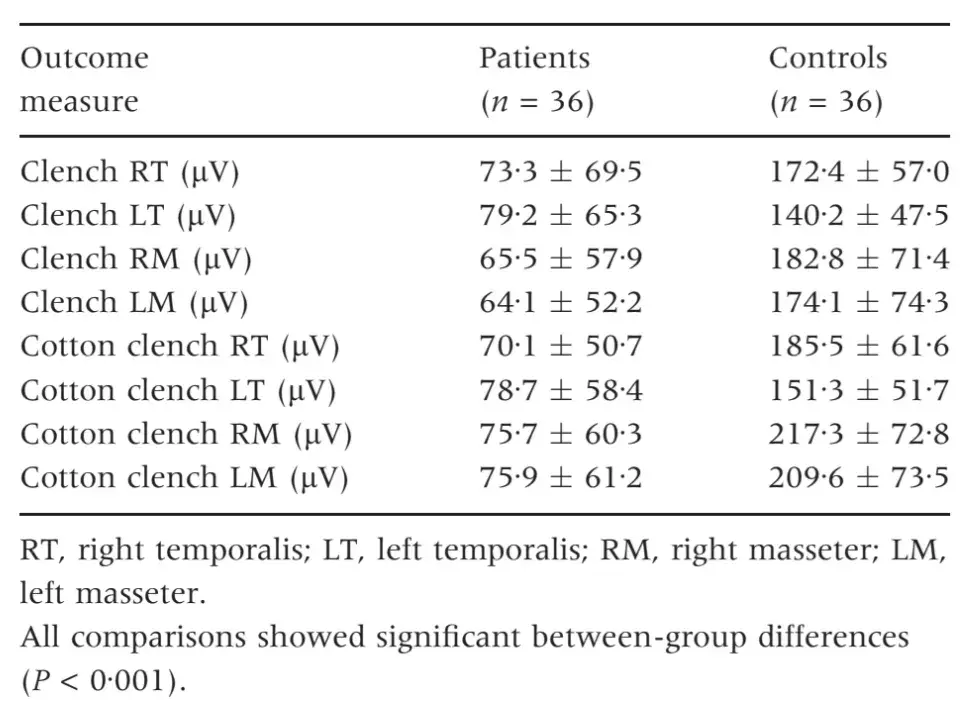
Table 4. Parameters of jaw movement and freeway space at rest. Comparison between patients and controls
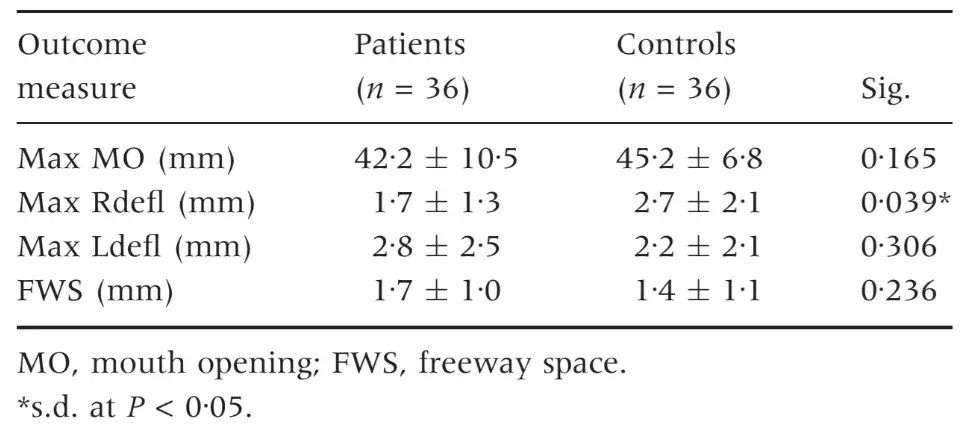
Table 5. Receiver operating characteristics (ROC) curve analysis for the main outcome parameters. AREA under the curve
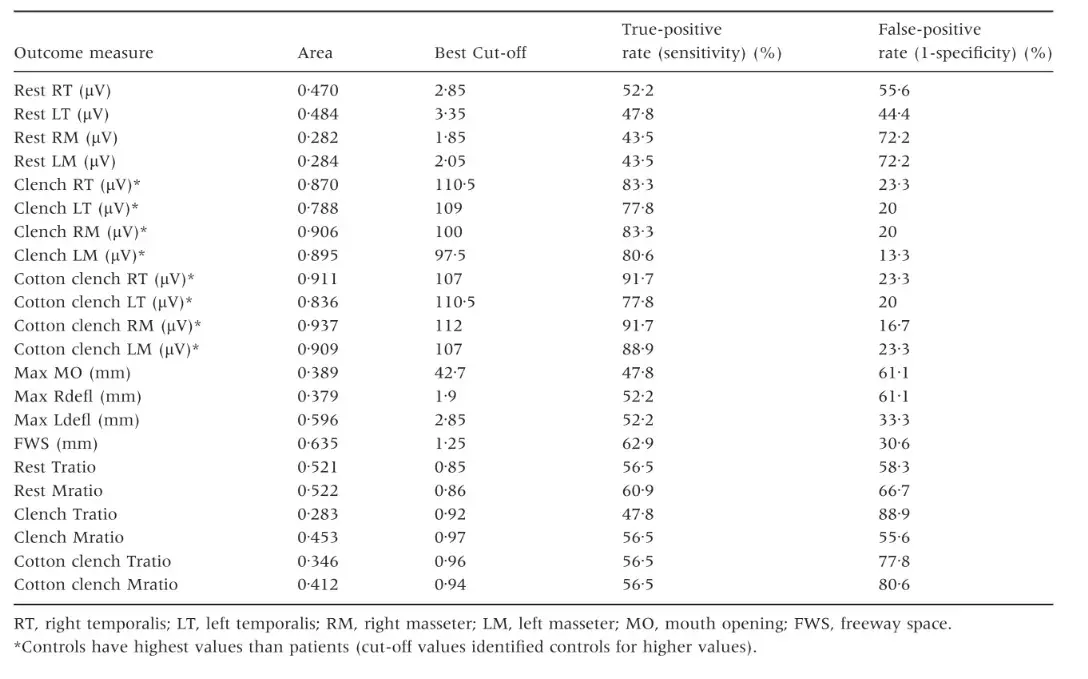
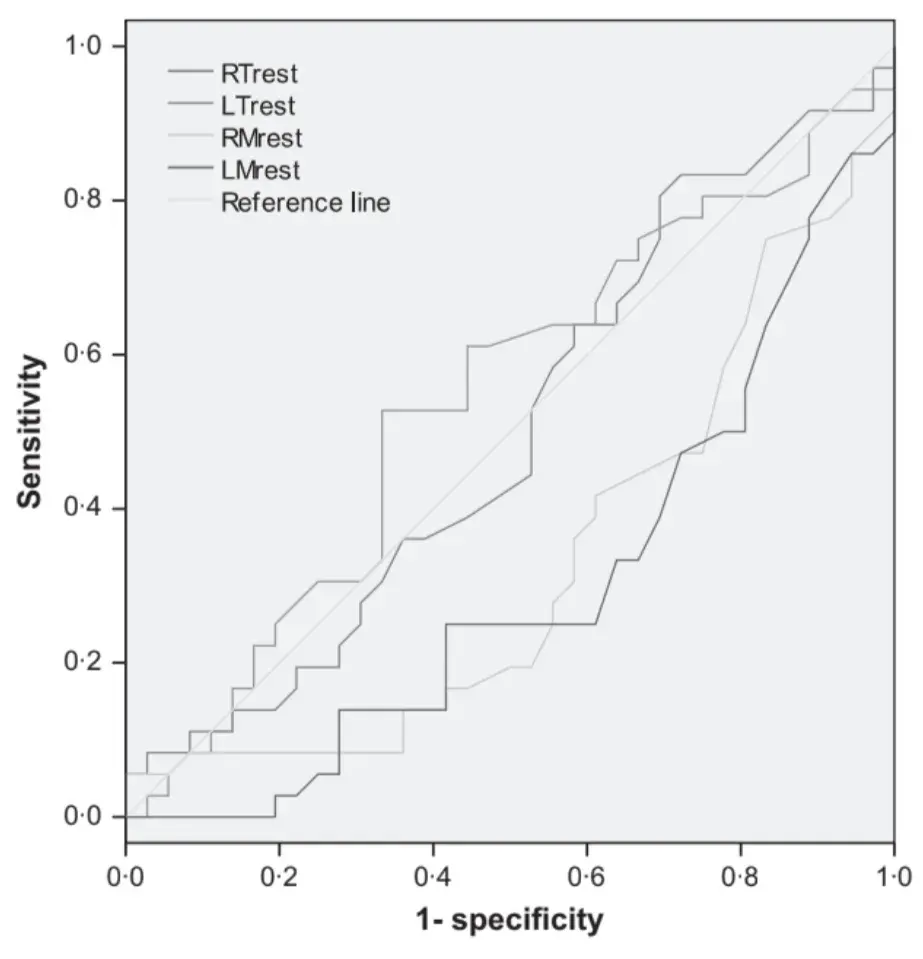 Fig. 1. Receiver operating characteristics curve analysis. Resting electromyography activity of the four investigated muscles.
Fig. 1. Receiver operating characteristics curve analysis. Resting electromyography activity of the four investigated muscles.
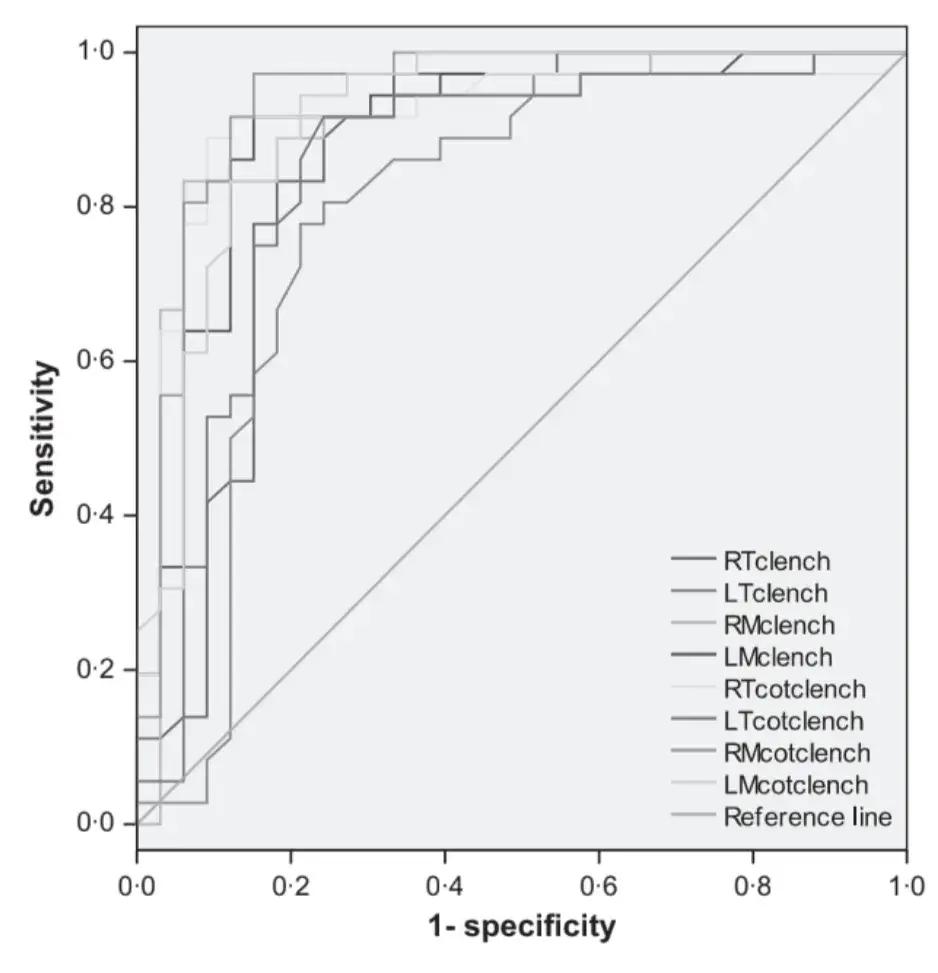 Fig. 2. Receiver operating characteristics curve analysis. Electromyography activity of the four investigated muscles during clenching tasks.
Fig. 2. Receiver operating characteristics curve analysis. Electromyography activity of the four investigated muscles during clenching tasks.
 Fig. 3. Receiver operating characteristics curve analysis. Kinesiographic parameters of jaw motion and interarch freeway space at rest.
Fig. 3. Receiver operating characteristics curve analysis. Kinesiographic parameters of jaw motion and interarch freeway space at rest.
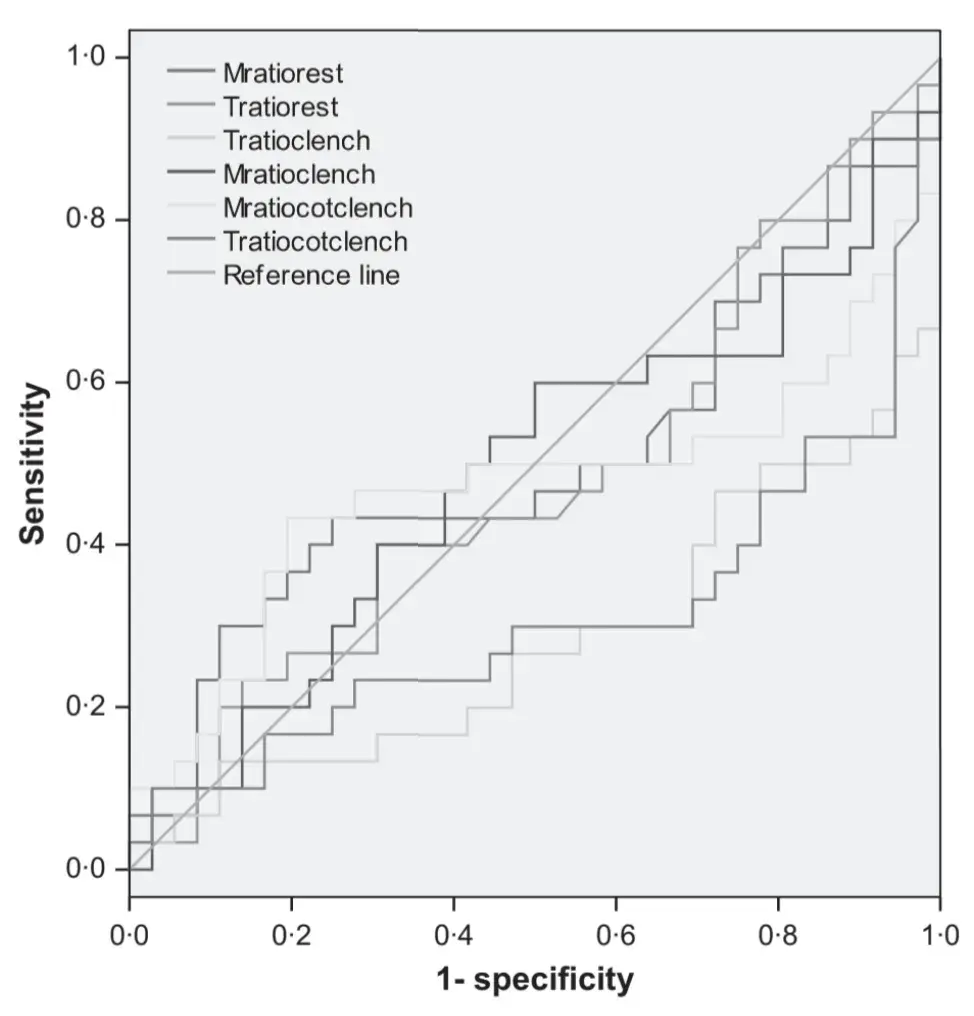 Fig. 4. Receiver operating characteristics curve analysis. Right:left electromyography activity ratio of the four investigated muscles.
Fig. 4. Receiver operating characteristics curve analysis. Right:left electromyography activity ratio of the four investigated muscles.
DISCUSSION
The accuracy of a diagnostic test is a critical issue to consider for the assessment of the clinical usefulness of any instruments and ⁄ or techniques purported to diagnose disease (14). The accuracy to diagnose disease as well as the ratios of false-positive and false-negative findings can be measured by comparing one test’s results with those of the standard of reference among diagnostic approaches, and a critical issue concerns the need to actually measure the main marker of disease.
While such an approach can be easily feasible for pathologies characterised by laboratory markers, it is much more complicated to adapt at painful syndromes, such as the TMD. Literature guidelines suggested that pain and limited jaw motion are the two main cardinal markers to identify TMD patients (15), thus supporting the adoption of clinical assessment via standardised examinations as the reference for TMD diagnosis (10, 16, 17). Notwithstanding that, the potential bias because of an over-rely on patients’ subjective experience to rate pain has been viewed as a matter of concern, and the search for strategies to give an objective evaluation of pain in TMD patients is still in progress (18, 19). In particular, the field of TMJ imaging researches allowed important achievements in recent years toward the attempt of gaining a better depiction of the link between clinical symptoms related to TMJ disorders and their related imaging signs (3, 11, 20–23). As concerns jaw muscle disorders, proposed diagnostic aids were based on the assessment of EMG activity and kinesiographic recordings of jaw movement patterns (24).
The available literature suggested that the diagnostic accuracy of electronic devices such as the sEMG and KG was much lower than that suggested by the manufacturers (4, 25). Notwithstanding that, the most striking finding from recent comprehensive literature reviews was that the number of researches on the argument was low and their quality poor (5, 6). The majority of well-designed studies adopted electronic devices that were strictly available to the involved research group and suggested that the EMG activity assessment has some potential additional value with respect to clinical examination alone (26, 27). In particular, the main parameter for an identification of patients with TMD-related pain was a reduced muscle force, viz. EMG activity, during clenching tasks (27). Such findings are in line with the pain adaptation model and its subsequent integration, postulating that pain leads to alterations in muscle activity aiming to limit movement and to protect the system against further injuries via a decreased activity of agonist muscles (28, 29). Thus, they are of interest mainly in the research setting because they provide attempts to quantify the reduction in muscle force when a painful condition is present, but an assessment of the actual clinical usefulness to measure electronically phenomena, viz. reduction in pain-related muscle force and eventual reduction of jaw mobility, which can be easily monitored clinically is questionable. Also, the issue of external validity of findings achieved on non-representative samples by the use of dedicated instruments not available for routine use is a matter of concern for many researches in the TMD field (30). These concerns assumed more importance if one considers that the actual validity of commercially available electronic devices, viz. their accuracy to detect TMD pain, has never been tested despite their long-lasting use in the clinical setting (9, 31).
Considering these drawbacks, the present investigation was designed to describe the accuracy of sEMG and KG to discriminate between patients with myofascial pain of jaw muscles and TMD-free asymptomatic subjects in a clinical setting.
Findings suggested that the only outcome measures with acceptable levels of sensitivity and specificity were related to parameters of EMG activity of masseter and temporalis muscles during clenching tasks. Temporomandibular disorder-free showed significantly higher EMG activity with respect to myofascial pain patients in maximum clenching on teeth and cotton rolls. By contrast, measures related to EMG activity at rest and to the ratio of EMG activity between symmetric muscles are poorly accurate to discriminate between patients and controls, with a true-positive rate below 60% and a false-positive rate ranging from about 44% to up to 89%. Also, the assessment of interarch freeway space endorsed unacceptable accuracy levels, viz. 63Æ5%. The examined kinesiographic parameters of jaw motion were the maximum mouth opening and the linearity of jaw opening along the mid-sagittal plane, and in both measurements, the between-group differences were not relevant.
Taken together, the findings supported the claims from reviews, suggesting that sEMG and KG, at present, may provide only ancillary documentation with respect to clinical diagnosis (4, 5, 32). EMG activity at rest cannot be used as a reliable parameter for myofascial pain diagnosis, because a cut-off value with concurrently acceptable levels of sensitivity and specificity could not be identified. In particular, the false-positive rate was high even if high levels of EMG activity were chosen as cut-off thresholds. The average rest EMG activity in the TMD-free group was higher than the cut-off value suggested by a group of practitioners with expertise in the use of the devices under investigation (i.e. 2Æ5 lV) (8) for all the four investigated muscles. At the single-patient level, this means that the adoption of such threshold as a stand-alone diagnostic criterion would have led to an incorrect classification of up to 86% of TMD-free subjects (31 of 36). Similar concerns might be raised for the measurement of the interarch freeway space and for kinesiographic parameters of jaw range of motion with a false-positive rate comprised between 30% and 60%. In particular, their theoretical validity is questionable, because KG recordings, even if their precision would be demonstrated, gave no advantages versus clinical assessment alone to measure clinically significant abnormalities of jaw range of motion (33).
The EMG activity during clenching tasks was significantly superior in the control group than in patients with myofascial pain, suggesting that EMG activity at maximum recruitment was two to three times higher in non-painful than in painful muscles. Such findings, along with findings that painful and non-painful muscles, have basically the same EMG activity at rest, corroborated the validity of the pain adaptation model (28). Thus, independently by the magnitude of EMG activity, which appears to be highly variable between studies depending on the raw electromyographic signal processing (7), there are enough elements to suggest that commercially available sEMG systems could replicate findings achieved in laboratory settings as concerns the pain–motor activity relationship (34).
Interesting findings also emerged as for the absence of significant gender differences in any of the outcome measures, thus suggesting that one of the potential points of concerns against the use of EMG in the clinical setting, viz. the need to validate different thresholds for EMG activity in men with respect to women (5), may be less important than believed. In any case, further investigations are strongly needed to support these findings and before managing data without sex adjustment in the clinical setting.
In view of the above considerations, it seems that literature cautionary statements against the routine use of sEMG and KG as diagnostic tools still remain valid. In particular, despite laudable efforts to test for these instruments repeatability and reliability (35, 36), the main concern related with the use of such devices is their lack of theoretical validity (37).
A scientifically sound premise to the use of a diagnostic tool is that it must be designed to measure the main indicator of disease, otherwise viewed as the main reason for patients to seek treatment. The present investigation showed that technological devices in the field of jaw muscle EMG and jaw motion recordings do not satisfy this basic requisite because they do not relate with the main clinical symptom, viz. pain, and they do not allow to add clinically significant information to those gathered with patients’ history taking and clinical assessment. Considering these premises, the need for an implementation of the quality of researches on the argument, with focus on the attempts to weigh all the potential bias for EMG recordings (i.e. among the others, facial morphology, bruxism, history, sex, age and body mass index), is strongly recommended. Also, in the clinical setting, the problem of overdiagnosis and subsequent overtreatment of TMD-free asymptomatic subjects is a matter of concern with the use of sEMG and KG as stand-alone diagnostic tools, and the biological, psychological, financial and social costs and consequences associated with overtreatment of non-treatment-needing subjects have become a focal point for contemporary medicine (38, 39).
Investigations attempting to use devices to measure pain-related features in TMD patients may have several potential points of methodological concerns at the design as well as at the strictly technical level. In this study, efforts have been made to minimise shortcomings. In the study design phases, a priori sample size calculation was a prerequisite to avoid type II errors, viz. false-negative findings. Actually, the statistical power of the investigation was lower than expected in the a priori calculation because the reference resting EMG values taken from the literature as the cut-off threshold (2Æ5 lV) were lower than the values recorded in this study. This means that an increase in the sample size would have been of benefit to achieve the actual 80% statistical power. Notwithstanding that, it may be argued that significant changes in the results could not be expected with an enlargement of the sample, because resting EMG values of asymptomatic subjects were on average higher than myofascial pain patients.
From a technical viewpoint, the adoption of personalised protocols must be considered the major limitation to generalisation of findings, so efforts have been made in this study to use commercially available devices and techniques in accordance with the protocols proposed by the manufacturers and, even more importantly, with examiners trained and calibrated with the use of such devices. Thus, it seems plausible that the risks FOR bias were minimised.
There are additional details about TMJ dysfunction diagnosis and treatment you can get in our course "Evidence-based treatment of TMJ dysfunction" by Jeffery P. Okeson, Ambra Michelotti and other experts.
CONCLUSIONS
The present investigation assessed the diagnostic accuracy of commercially available devices for sEMG and KG recordings for myofascial pain. Resting sEMG values, symmetry of muscle activity at rest and during clenching tasks, KG parameters of jaw range of motion and the measurement of the interarch vertical freeway space showed high rates of false-positive findings in TMD-free subjects, with a percentage ranging from 30Æ6% to 88Æ9%.
Surface electromyography activity during clenching tasks showed a significantly higher activation in TMD-free subjects, in line with the pain adaptation model and with previous research findings, thus suggesting that patterns of muscle activation governing the pain–motor function relationship are not dependent on the absolute magnitude of activity and on the type of recording techniques.
The clinical implications of these findings are important: muscle activity levels and jaw movement aberrations cannot be used as proxies for muscle pain because of the fact that there were no significant differences between groups regarding mean values of sEMG and KG results. Clinically, it implies that one cannot use these instruments to either diagnose or monitor the course of TMD in a single individual.
While this may be disappointing to some, it is no different than the problem facing physicians who treat other pain conditions. Thus, sEMG and KG assessments cannot be proposed as instruments to either diagnose or monitor the course of disease with respect to baseline diagnostic data, in line with the physicians’ approach to other pain conditions (e.g. back pain, headache disorders and fibromyalgia). In conclusions, the use of sEMG and KG devices as diagnostic tools for patients with myofascial pain of jaw muscles must be discouraged because of the potential risk for overdiagnosis and overtreatment.
Authors:
D. MANFREDINI, F. COCILOVO, L. FAVERO, G. FERRONATO, S. TONELLO, L. GUARDA-NARDINI
References
McNeill C. Management of temporomandibular disorders: concepts and controversies. J Prosthet Dent. 1997;77:510–522.
American Association for Dental Research. AADR TMD policy statement revision.
Petersson A. What you can and cannot see in TMJ imaging – an overview related to the RDC ⁄ TMD diagnostic system. J Oral Rehabil. 2010;37:771–778.
Lund JP, Widmer CG, Feine JS. Validity of diagnostic and monitoring tests used for temporomandibular disorders. J Dent Res. 1995;74:1133–1143.
Klasser GD, Okeson J. The clinical usefulness of surface electromyography in the diagnosis and treatment of temporomandibular disorders. J Am Dent Assoc. 2006;137:763–771.
Suvinen TI, Kemppainen P. Review of clinical EMG studies related to muscle and occlusal factors in healthy and TMD subjects. J Oral Rehabil. 2007;34:631–644.
Baba K, Ono Y, Clark GT. Instrumental approach. In: Manfredini D, ed. Current concepts on temporomandibular disorders. Berlin: Quintessence Publishing, 2010:223–236.
Cooper BC. Parameters of an optimal physiological state of the masticatory system: the results of a survey of practitioners using computerized measurement devices. Cranio. 2004;22: 220–233.
Cooper BC, Kleinberg I. Establishment of temporomandibular physiological state with neuromuscular orthosis treatment affects reduction of TMD symptoms in 313 patients. Cranio. 2008;26:104–117.
Dworkin SF, Leresche L. Research diagnostic criteria for temporomandibular disorders: review, criteria examinations and specifications, critique. J Craniomandib Disord Fac Oral Pain. 1992;6:301–355.
Manfredini D, Guarda-Nardini L. Agreement between research diagnostic criteria for temporomandibular disorders and magnetic resonance diagnoses of temporomandibular disc displacement in a patient population. Int J Oral Maxillofac Surg. 2008;37:612–616.
Manfredini D, Piccotti F, Ferronato G, Guarda-Nardini L. Age peaks of different RDC ⁄ TMD diagnoses in a patient population. J Dent. 2010;38:392–399.
Metz CE. Basic principles of ROC analysis. Sem Nuc Med. 1978;8:283–298.
Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM; Cochrane Diagnostic Test Accuracy Working Group. Systematic review of diagnostic test accuracy. Ann Intern Med. 2008;149:889–897.
Manfredini D. Fundamentals of TMD management. In: Manfredini D, ed. Current concepts on temporomandibular disorders. Berlin: Quintessence Publishing, 2010:305–318.
Okeson JP. Management of temporomandibular disorders and occlusion, 6th edn. St Louis: Elsevier-Mosby, 2008.
De Leeuw R. The American Academy of Orofacial Pain. Orofacial pain: guidelines for assessment, diagnosis, and management. Chicago: Quintessence Publishing, 2008.
Steenks MH, de Wijer A. Validity of the research diagnostic criteria for temporomandibular disorders axis I in clinical and research settings. J Orofac Pain. 2009;23:9–16.
Ohrbach R. Disability assessment in temporomandibular disorders and masticatory system rehabilitation. J Oral Rehabil. 2010;37:452–480.
Manfredini D, Tognini F, Zampa V, Bosco M. Predictive value of clinical findings for temporomandibular joint effusion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:521–526.
Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL et al. Research diagnostic criteria for temporomandibular disorders (RDC ⁄ TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:844–860.
Koh KJ, List T, Petersson A, Rohlin M. Relationship between clinical and magnetic resonance imaging diagnoses and findings in degenerative and inflammatory temporomandibular joint diseases: a systematic literature review. J Orofac Pain. 2009;23:123–139.
Manfredini D, Guarda-Nardini L. Ultrasonography of the temporomandibular joint: a literature review. Int J Oral Maxillofac Surg. 2009;38:1229–1236.
Jankelson B. Neuromuscular aspects of occlusion: effects of occlusal position on the physiology and dysfunction of the mandibular musculature. Dent Clin North Am. 1979;23:157–168.
Greene CS. The role of biotechnology in TMD diagnosis. In: Laskin DM, Greene CS, Hylander WL, eds. TMDs. An evidence-based approach to diagnosis and treatment. Chicago: Quintessence Publishing, 2006:193–202.
Bode´re´ C, Te´a SH, Giroux-Metges MA, Woda A. Activity of masticatory muscles in subjects with different orofacial pain conditions. Pain. 2005;116:33–41.
Tartaglia GM, Moreira Rodrigues da Silva MA, Bottini S, Sforza C, Ferrario VF. Masticatory muscle activity during maximum voluntary clench in different research diagnostic criteria for temporomandibular disorders (RDC ⁄ TMD) groups. Man Ther. 2008;13:434–440.
Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–694.
Murray GM, Peck CC. Orofacial pain and jaw muscle activity: a new model. J Orofac Pain. 2007;21:263–278.
Palla S, Farella M. External validity: a forgotten issue? Int J Prosthodont. 2010;23:293–294.
Jankelson B, Radke J. The myomonitor: its use and abuse. Quintessence Int Dent Digest. 1978;9:35–39, 47–52.
Mohl ND. Reliability and validity of diagnostic modalities for temporomandibular disorders. Adv Dent Res. 1993;7:113–119.
Dworkin SF, Leresche L, DeRouen T, von Korff MR. Assessing clinical signs of temporomandibular disorders: reliability of clinical examiners. J Prosthet Dent. 1990;63:574–579.
Svensson P, Wang K, Sessle BJ, Arendt-Nielsen L. Associations between pain and neuromuscular activity in the human jaw and neck muscles. Pain. 2004;109:225–232.
Castroflorio T, Bracco P, Farina D. Surface electromyography in the assessment of jaw elevator muscles. J Oral Rehabil. 2008;35:638–645.
Suvinen TI, Malmberg J, Forster C, Kemppainen P. Postural and dynamic masseter and anterior temporalis muscle EMG repeatability in serial assessments. J Oral Rehabil. 2009;36: 814–820.
Dao TTT, Feine JS, Lund JP. Can electrical stimulation be used to establish a physiologic occlusal position? J Prosthet Dent. 1988;60:509–514.
Greene CS. Science transfer in orofacial pain. In: Lund JP, Lavigne GJ, Dubner R, Sessle BJ, eds. Orofacial pain. From basic science to clinical management. Chicago: Quintessence Publishing, 2008:281–286.
Manfredini D. Integration of research into the clinical practice. In: Manfredini D, ed. Current concepts on temporomandibular disorders. Berlin: Quintessence Publishing, 2010:459–468.


