Effectiveness of Supportive Care in the Prevention of Peri-implant Diseases in Brånemark Protocol Rehabilitations: A Retrospective Cohort Study
Abstract
This retrospective cohort study aimed to evaluate the efficiency of supportive periodontal treatment (SPT) on peri-implant disease prevention. A total of 63 rehabilitated patients with 504 implants were selected with both maxillary and mandibular Brånemark-type protocols (all-on-four system) placed between 2004 and 2014 in a private practice with 5 to 15 years of follow-up. Further details about All-on-4 rehabilitation protocols are accessible for you to learn on our course "Сhallenges of the All-On-4 total rehabilitation" by Paulo Malo and team.
Study participants were divided into those who adhered to SPT with regular intervals of two or more times a year (Group 1) and those with irregular intervals with more than 1 year without attending SPT (Group 2). The implants placed were evaluated clinically and radiographically to assess peri-implant diseases. The prevalence of mucositis and peri-implantitis in the total population was 64.7% (326 implants) and 3.7% (19 implants), respectively. Group 2 had higher rates of mucositis (181 implants) and peri-implantitis (16 implants) than Group 1 (145 and 3 implants, respectively) (P < .05). The odds ratio for peri-implantitis in Group 2 was 7.1. The results suggest that patients who regularly received SPT had lower chances of developing peri-implant diseases.
Introduction
Osseointegrated dental implants are widely used in oral rehabilitations in partially and fully edentulous patients. The Brånemark “all-on-four” technique guarantees good support and predictability for an immediate fixed prosthesis in edentulous arches(1,2) ; however, the high prevalence rate of peri-implant diseases in cases treated with this method has already been widely reported in the literature.(3–7) Therefore, the monitoring of patients undergoing this type of rehabilitation is essential. By incorporating peri-implant supportive therapies, it is possible to prevent peri-implant diseases and ensure successful osseointegration.(6–9)
It is important to highlight that there are currently no scientific evidence-based guidelines available in the literature that ensure the prevention of peri-implant diseases.(9) However, the cause-and-effect relationship between biofilm accumulation and peri-implant diseases has already been confirmed.(10) Furthermore, various factors related to implants, dentists, and patients can contribute to the severity of the disease and can be identified and controlled through an organized, professional monitoring program.(9)
Training sessions with patients to teach them to optimize oral hygiene, implementing techniques for decontamination of the implant surface, and regularly monitoring peri-implant clinical parameters can be considered essential measures for reducing the rate of peri-implant disease.(11,12) The absence of these measurements can be considered as a risk factor for the disease.(9,13)
Reliable clinical parameters have been used for diagnosing peri-implant diseases.(11,14) Among them, bleeding on probing, visible plaque, and calculus index15 are valuable tools by which to evaluate the disease activity and patient hygiene habits and to identify the presence and location of calcified biofilm.(11,16)
In addition, the peri-implant probing depth in conjunction with radiographic analysis can help identify bone loss(11,13,14,17) and assist in the differential diagnostic examination for gingival hyperplasia.(11,13,14)
Systematic reviews have linked the effects of supportive periodontal treatment (SPT) to both implant survival rates and the prevalence of peri-implant diseases.(18–20) A potential improvement of peri-implant health has been reported among patients who adhered to SPT during a maintenance period after implant placement.
Based on this context, this retrospective cohort study aims to evaluate the efficiency of SPT in the prevention of mucositis and peri-implantitis in patients rehabilitated with Brånemark-type protocols.
Materials and Methods
The Institutional Ethics in Research Committee of the Universidade Veiga de Almeida (Rio de Janeiro, Brazil) approved the study design (code: CAAE 64357916.3.0000.5289). The study was performed in accordance with the 1975 Declaration of Helsinki and was conducted as a retrospective cohort following the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.(21) All patients provided written informed consent for their inclusion in the study.
Study Population
The study participants were selected from a consecutive list of patients seen at a private dental clinic between 2004 and 2014 and who were subjected to immediate all-on-four Brånemark treatment protocols. The patients underwent implant placement in the maxilla and mandible in a single surgical operation using the immediate loading technique. The teeth considered lost were extracted and, after bone regularization, four implants were placed in accordance with the Brånemark “all-on-four” technique. All surgical procedures were conducted by the same surgeon (A.V.). The implants used were standardized with a Morse cone prosthetic connection and mini pillar-type intermediates. All patients had a mold taken immediately after surgery and received a provisional, fixed resin prosthesis one day later. Six months after implant placement, patients received the final metal-ceramic fixed prosthesis, made in the same prosthetic laboratory.
Immediately after placing the provisional prostheses, patients were enrolled in the SPT program, wherein they received instructions and guidelines regarding complete oral hygiene of their prostheses. Patients were encouraged to attend dental appointments every 6 months to receive the appropriate clinical SPT procedures, such as prosthesis removal, having the clinical parameters of disease diagnosis recorded, and proper hygiene maintenance of the prostheses, implants, and oral cavity.
Inclusion Criteria
Patients of either sex were eligible for inclusion in the study if they met the following criteria: (1) had received maxillary and mandibular implant-supported fixed prostheses by the Brånemark “all-on-four” system inserted as part of the oral hygiene maintenance and control program performed in a private clinic; (2) began and completed treatment at the same location, were operated on by the same surgeon, used the same system of implants, and received prostheses with the same manufacture pattern from the same prosthetic laboratory; (3) had prostheses with good occlusal balance, verified via 200-µm carbon Articulating Paper (Bausch); and (4) had received implants between 2004 and 2014.
Exclusion Criteria
Patients were excluded from the study if they presented with any of the following: (1) systemic diseases, such as acquired immunodeficiency syndrome, leukemia, cirrhosis, uncontrolled diabetes mellitus, and hepatitis; (2) a history of rheumatic fever or the need for antibiotic prophylaxis in the case of patients with artificial heart valves; (3) the use of antibiotic prophylaxis within the previous 2 months; (4) pregnancy or use of hormone therapy; or (5) a need for more than four implants to rehabilitate one of the arches.
Peri-implant SPT
The peri-implant SPT visits proposed for the patients occurred at least twice a year, adhering to the following protocol: (1) removal of the maxillary and mandibular prostheses(11); (2) clinical inspection to register the peri-implant parameters; (3) using a plastic curette to remove dental calculus, and irrigation with antiseptic if necessary(11,12,22); (4) prophylaxis with a bicarbonate jet to remove biofilm from the supra- and subgingival environment(11,12,14); (5) repair of the prosthesis, if necessary, and conducting ultrasound and bicarbonate jet cleaning to remove calculus, plaque, and pigmentation together, as well as polishing it with a specific material to remove irregularities, smoothing the surface, and soaking it in 0.12% chlorhexidine (PerioGard, Colgate) before replacement in the mouth; (6) subsequent replacement and adjustment to maintain occlusal balance; (7) reinforcement of the previously provided oral hygiene instructions; and (8) delivering instruments (such as soft, wire, and compatible interdental brushes) to continue good hygiene of the prostheses and implants between appointments.
Peri-implant Parameters Assessed
Two periodontists (V.B.R. and C.V.) clinically evaluated the patients who received SPT, respecting the frequency routine of each one (kappa and kappa weighted values were both > 0.85). The parameters chosen for evaluation and documentation by periodontists in patient records include viable plaque index, dental calculus index, bleeding on probing, peri-implant probing depth, and radiographic evaluations. These parameters were selected primarily from prior clinical studies of peri-implant evaluation.(11,13,14,17)
Visible Plaque Index
Proposed by Mombelli et al,(15) the Plaque Index was evaluated as no plaque (0 points) or the presence of thick visible plaque (1 point).
Dental calculus index
The presence or absence of calcified biofilm was identified according to the absence of dental calculus (0 points) or the presence of supragingival and/or subgingival calculus (1 point). Notably, identifying the presence of dental calculus is one more tool for the clinician to institute the necessary treatment measures for implant maintenance and to locate areas marked by greater difficulty of access to hygiene and showing an increased risk of disease development.
Bleeding on probing
The bleeding index was determined using a probe and scored for the absence of bleeding (0 points) or the presence of bleeding at the examined point (1 point). The bleeding index determined at the probe was used to diagnose mucositis in the implants. The presence of bleeding after delicate probing in at least one site per implant was sufficient enough to classify the implant as being complicated by mucositis.
Peri-implant probing depth
The examiners used probes to record the probing depth in millimeters. Peri-implant probing depths ≥ 5 mm suggested bone loss at the corresponding site. If the radiograph confirmed bone loss at the region, the implant was classified as complicated by peri-implantitis.
Radiographic evaluation
Periapical radiographs were taken using the long cone technique, parallel to the implant, in cases where the probing depth was ≥ 5 mm (to establish the differential diagnosis between bone loss and gum hyperplasia) or in cases where the implant thread exposure was clinically verified (suggesting loss of osseointegration with retraction of the peri-implant mucosa).
Data from Patient Records
The records from 63 patients (504 implants) who met the inclusion criteria were selected and divided into the following groups: patients with both maxillary and mandibular Brånemark all-on-four protocols who attended the SPT program two or more times a year (Group 1) and patients with both maxillary and mandibular Brånemark all-on-four protocols who participated in the SPT program at irregular intervals and went more than 1 year without attending the SPT (Group 2).
Sample Calculation and Statistical Analysis
To analyze the sample’s power, G*Power software (version 3.1.9.4) was used to compare proportions of peri-implantitis cases between independent Groups 1 and 2. The effect size (w) was established in 0.3, the power of the test was 0.80, and an alpha value of .05 was established. The results suggested 141 implant recipient sites would be needed. SPSS software (version 25, IBM) was used to conduct descriptive analyses, while proportional comparisons were performed using chisquare test with a significance level established at P = .05.
Results
Sixty-three subjects who fulfilled the inclusion criteria (67% women, 21% men) were included in the study, and a total of 504 implants were evaluated. Subject distribution was homogeneous in both Group 1 (35 subjects) and Group 2 (28 subjects) (P = .37). There were no differences between the groups concerning patient age (95% were older than 50 years old), smoking history, diabetes history, existence of preexisting periodontal diseases, and parafunctional habits (Table 1). The presence of preexisting periodontal disease before prosthetic rehabilitation with implants was observed in 33 subjects (52%), including 19 in Group 1 and 14 in Group 2, with no statistical difference apparent between them (P = .387). However, 30 subjects lost their implants due to causes other than periodontal disease (48%).
The numbers of patients with visible plaque, dental calculus, bleeding on probing, and peri-implantitis are presented in Table 1. Of the 504 analyzed implants, 400 presented with visible plaque in at least one site, totaling 79.4% of the study population’s implants. Of the 400 implants, there were no differences between the findings in Group 1 (191 implants; 47.8%) and Group 2 (209 implants; 52.3%) concerning this parameter (P = .368).
Evaluation of the dental calculus also revealed no statistical differences, with 57 (43.2%) and 75 (56.8%) implants affected in Groups 1 and 2, respectively. Regarding bleeding on probing, 326 implants presented positive bleeding in at least one site evaluated (64.68% of the total population). Group 2 showed a higher rate of bleeding/mucositis (181 implants; 55.5%) than Group 1 (145 implants; 44.5%) (P = .04).
The implant aspect with the greatest probing depth was recorded. Nineteen implants (3.7% of the sample) presented probing depths ≥ 5 mm, as confirmed by radiographs, and were classified as peri-implantitis cases. Of these, 16 belonged to Group 2, with a higher prevalence than Group 1 (3 implants) (P = .003). The odds ratio of patients in Group 2 showing peri-implantitis was 7.1.
In this sample, there was no correlation between the occurrence of mucositis and peri-implantitis and the patient variables smoking history, diabetics, preexisting periodontal diseases, and parafunctional habits (P > .05).
Table 1 Patient and Implant Characteristics of Both Groups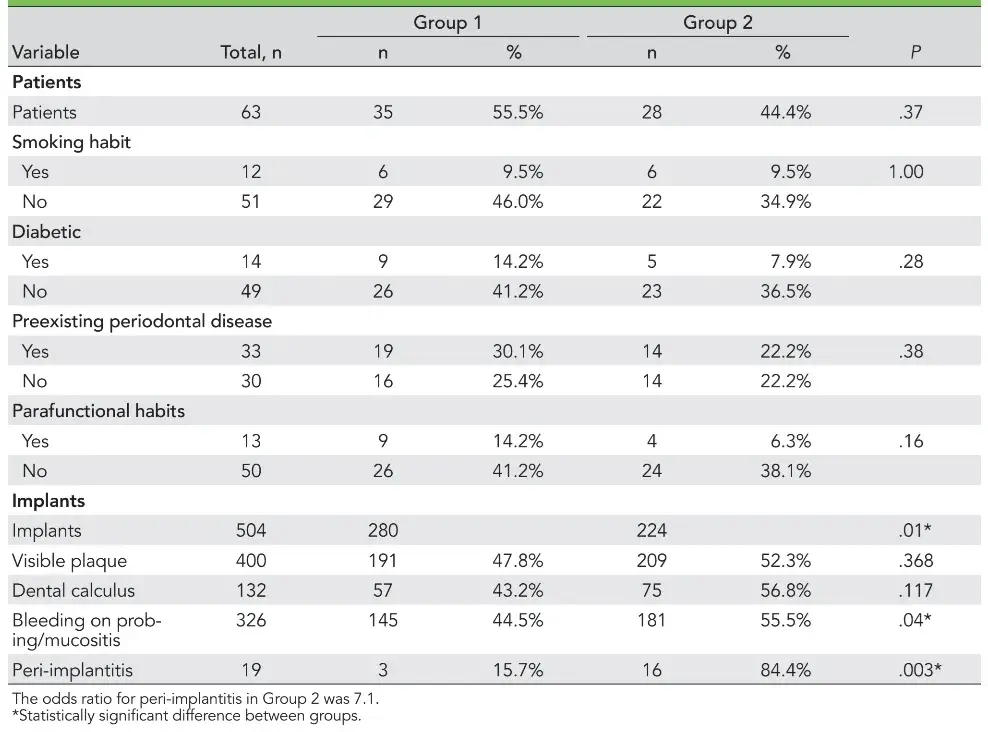
Discussion
A series of anatomical differences between periodontal and peri-implant tissues justify the existence of greater fragility in the peri-implant biologic seal. The disposition of the collagen fibers and the reduced vascularization in the region denotes a greater degree of fragility of these implants to peri-implant diseases,(23) justifying the need to pay special attention to implant management. Patients who are completely edentulous, with reduced bone availability and rehabilitated with a prosthesis or implants, must be even more vigilant, as the presence of peri-implant diseases followed by bone loss can progress with implant failure, making it unfeasible to maintain the prosthetic rehabilitation with hygiene alone.(1,2) Prostheses with flanges in acrylic resin, even in ideal conditions of regular hygiene, require close attention for adequate oral hygiene. The periodic maintenance of this prosthesis type can contribute to the prevention of peri-implant diseases.(9) In this context, the present retrospective cohort study assessed the impact of SPT on preventing mucositis and peri-implantitis in patients rehabilitated with Brånemark-type protocols. Various potential risk indicators have been associated with peri-implant diseases and bone loss.(6,7)
Among them, patient-related factors include smoking, diabetes, previous periodontal diseases, and parafunctional habits.(3,10,24) However, in the present study sample, there was no correlation between these cited factors and peri-implant disease development.
Furthermore, implant- and prosthesis-related factors (such as the design of the implant–abutment complex and implant microgeometry(25,26)) and surgical factors (including surgical technique and surgeon ability(27) ) are variables that are involved with implant success and maintenance. One strong point of the present study was the ability to analyze a homogeneous sample. This was achieved by standardizing the system and microgeometry of the implants placed over the years by the same surgeon. Additionally, the use of the same abutment systems and prosthesis laboratory helped control the biases of the study sample.
It has previously been suggested that a direct relationship exists between clinical parameters such as plaque scores, dental calculus, and bleeding on probing and a higher risk of developing peri-implantitis.(13)
In the present study, however, Group 2 presented an insignificant increase in all these parameters relative to Group 1. This absence of a significant difference may suggest a limitation of this study, probably caused by the reduced prevalence of diseased sites for an observational retrospective study to measure the disease’s prevalence. The prevalence of mucositis in the present study sample (64.68%) was significantly higher than that of peri-implantitis (3.7%). This result is in accordance with a study evaluating the prevalence of peri-implant diseases of 212 partially edentulous subjects rehabilitated with osseointegrated implants, which reported rates of 64.6% and 8.9% for mucositis and peri-implantitis, respectively.(3) The 2012 European Academy of Osseointegration consensus estimated that peri-implantitis affects 10% of implants within 5 to 10 years after implant placement.(28) However, a vast range of prevalence rates are reported in the literature, arising partly due to varying implant designs and differences in the thresholds applied for bone loss and soft tissue research. The present study suggested peri-implantitis prevalence rates of 3.76% in the overall study population (19 implants), 0.59% in Group 1 (3 implants), and 3.17% in Group 2 (16 implants), suggesting the effectiveness of SPT in the prevention of peri-implantitis. These findings can be considered lower than those of previous studies.(7,13) Costa et al(13) reported high rates of peri-implantitis: 18.0% and 43.9% in patients with and without preventive maintenance, respectively. However, Costa et al evaluated a sample of partially edentulous individuals, and it is crucial to consider that the teeth are potential pathogen pathways.(15,29)
The present study shows a divergence from previous systematic reviews concerning the effects of SPT in preventing peri-implant diseases and implant loss.(9,18,19,30)
According to Hultin et al,(30) few available studies have investigated the long-term effects of supportive programs for implant patients. In contrast, Ramanauskaite and Tervonen(19) suggested that, to prevent peri-implantitis, the introduction of an individually supportive program based on patient motivation and reinstruction in oral hygiene combined with professional implant cleaning is crucial. Further, Lin et al(18) emphasized that a supportive program can potentially improve patient peri-implant health. According to Tomasi and Derks,(31) no study assessing the incidence and prevalence of peri-implant diseases can be considered as providing gold-standard recommendations so far. Previous studies show failures in the convenience samples and failures in determining the correct diagnosis of mucositis, peri-implant, and peri-implant health (eg, peri-implant diseases are influenced by different factors related to the patients, types of implant systems, and/or types of prostheses). Further, there are flaws in the observation and follow-up methods for evaluating the appearance of cases.(4,7)
Based on the data obtained in the literature and from the present population study, it can be stated that patients who do not adhere to a strict peri-implant maintenance program are more susceptible to the appearance of disease than individuals who are compliant with a maintenance program.(6,10) Despite the longer dental appointments caused by SPT due to the prostheses removal, clinical inspection, and prophylaxis, which require the prosthetic screws to be replaced at shorter intervals of time, these disadvantages are overcome by the peri-implant disease prevention and better long-term results. Professional management could intervene early in the initial stage to prevent or limit disease progression and facilitate improved understanding among patients to perform the correct methods of oral hygiene.(9,11,12)
As peri-implant disease develops slowly and gradually, it is imperative to make an early diagnosis and introduce prompt treatment to prevent the destruction of peri-implant protective and supportive tissues.(6,14,23) In modern dental treatment procedures, including those with regular individualized maintenance, peri-implantitis cases are rare because the disease is typically intercepted early.
Conclusions
Within the limits of the present study, the data suggest that patients who regularly participate in an SPT program (at least twice a year) have a lower chance of developing peri-implant diseases.
You have the opportunity to gather more in-depth information about Peri-implant diseases treatment and prevention in our course "Implantation without peri-implantitis".
Acknowledgments
The authors declare no conflicts of interest.
List of authors:
Suelen Cristina Sartoretto, Vivian Berti Ramos, Eduardo Jose Veras Lourenço, Avelino Veit, Cynthia Veit, Luis Felipe Jochims Schneider, Larissa Maria Cavalcante
References
Özdemir Dog˘ an D, Polat NT, Polat S, S¸ eker E, Gül Eb. Evaluation of “all-on-four” concept and alternative designs with 3D finite element analysis method. Clin Implant Dent Relat Res 2014;16:501–510.
Patzelt SB, Bahat O, Reynolds MA, Strub JR. The all-on-four treatment concept: A systematic review. Clin Implant Dent Relat Res 2014;16:836–855.
Ferreira SD, Silva GL, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol 2006;33:929–935.
Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol 2008;35:286–291.
Koldsland OC, Scheie AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol 2010;81:231–238.
Mombell A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res 2012;23:67–76.
Atieh MA, Alsabeeha NH, Faggion CM Jr, Duncan WJ. The frequency of peri-implant diseases: A systematic review and meta-analysis. J Periodontol 2013;84:1586–1598.
Balshi TJ, Wolfinger GJ, Slauch RW, Balshi SF. A retrospective analysis of 800 Brånemark System implants following the All-on-Four protocol. J Prosthodont 2014;23:83–88.
Monje A, Aranda L, Diaz KT, et al. Impact of maintenance therapy for the prevention of peri-implant diseases: A systematic review and meta-analysis. J Dent Res 2016;95:372–379.
Heitz-Mayfield LJ. Peri-implant diseases: Diagnosis and risk indicators. J Clin Periodontol 2008;35:292–304.
Todescan S, Lavigne S, Kelekis-Cholakis A. Guidance for the maintenance care of dental implants: Clinical review. J Can Dent Assoc 2012;78:c107.
Smeets R, Henningsen A, Jung O, Heiland M, Hammächer C, Stein JM. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med 2014;10:34.
Costa FO, Takenaka-Martinez S, Cota LO, Ferreira SD, Silva GL, Costa JE. Peri-implant disease in subjects with and without preventive maintenance: A 5-year follow-up. J Clin Periodontol 2012;39:173–181.
Salvi GE, Lang NP. Diagnostic parameters for monitoring peri-implants conditions. Int J Oral Maxillofac Implants 2004;19:116–123.
Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol 1987;2:145–151.
Lang NP, Nyman S, Senn C, Joss A. Bleeding on probing as it relates to probing pressure and gingival health. J Clin Periodontol 1991;18:257–261.
Gay IC, Tran DT, Weltman R, et al. Role of supportive maintenance therapy on implant survival: A university-based 17 years retrospective analysis. Int J Dent Hyg 2016;14:267–271.
Lin CY, Chen Z, Pan WL, Wang HL. The effect of supportive care in preventing peri-implant diseases and implant loss: A systematic review and meta-analysis. Clin Oral Implants Res 2019;30:714–724.
Ramanauskaite A, Tervonen T. The efficacy of supportive peri-implant therapies in preventing peri-implantitis and implant loss: A systematic review of the literature. J Oral Maxillofac Res 2016;7:e12.
Salvi GE, Zitzmann NU. The effects of anti-infective preventive measures on the occurrence of biologic implant complications and implant loss: A systematic review. Int J Oral Maxillofac Implants 2014;29:292–307.
Plos Medicine Editors. Observational studies: Getting clear about transparency. PLoS Med 2014;11:e1001711.
Figuero E, Graziani F, Sanz I, Herrera D, Sanz M. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000 2014;66:255–273.
Wang Y, Zhang Y, Miron RJ. Health, maintenance, and recovery of soft tissues around implants. Clin Implant Dent Relat Res 2016;18:618–634.
Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: A review of the literature. Clin Oral Implants Res 2002;13:1–19.
Albouy JP, Abrahamsson I, Persson LG, Berglundh T. Spontaneous progression of peri-implantitis at different types of implants. An experimental study in dogs. I: Clinical and radiographic observations. Clin Oral Implants Res 2008;19:997–1002.
Laurell L, Lundgren D. Marginal bone level changes at dental implants after 5 years in function: A meta-analysis. Clin Implant Dent Relat Res 2011;13:19–28.
Poli PP, Beretta M, Grossi GB, Maiorana C. Risk indicators related to peri-implant disease: An observational retrospective cohort study. J Periodontal Implant Sci 2016;46:266–276.
Klinge B, Meyle J. Peri-implant tissue destruction. The Third EAO Consensus Conference 2012. Clin Oral Implants Res 2012;23:108–110.
Hultin M, Gustafsson A, Hallström H, Johansson LA, Ekfeldt A, Klinge B. Microbiological findings and host response in patients with peri-implantitis. Clin Oral Implants Res 2002;13:349–358.
Hultin M, Komiyama A, Klinge B. Supportive therapy and the longevity of dental implants: A systematic review of the literature. Clin Oral Implants Res 2007;18:50–62.
Tomasi C, Derks J. Clinical research of peri-implant diseases—Quality of reporting, case definitions and methods to study incidence, prevalence and risk factors of peri-implant diseases. J Clin Periodontol 2012;39(suppl 12):207–223.


