An in vitro study into three different PRF preparations for osteogenesis potential
Abstract
Objective:
To investigate the effect of Advanced Platelet-Rich Fibrin (A-PRF+), Leukocyte Platelet-Rich Fibrin (L-PRF), and injectable Platelet-Rich Fibrin (i-PRF) on osteogenesis of a human osteoblast-like cell line in vitro.
Background:
Different PRF protocols are used in clinical dentistry in the last years. Recent literature documented the positive impact of PRF derivatives in vivo and in vitro, on different types of cells. However, hardly any literature comparing the new protocols for PRF (the A-PRF+ and i-PRF) with the original protocol of PRF (L-PRF) is present for osteoblast-like cells.
Materials and Methods:
A-PRF+, L-PRF, and i-PRF were prepared from six male donors and pre-cultured with 10 mL culture medium for 6 days. 5 x 103 cells/ml osteoblasts from the osteoblast cell line (U2OS) were seeded and cultured either with conditioned medium derived from the different PRF conditions or with regular culture medium. At five different time points (0, 7, 14, 21, 28 days), the osteogenic capacity of the cells was assessed with Alizarin Red S to visualize mineralization. Also in these cells, the calcium concentration and alkaline phosphatase activity were investigated. Using qPCR, the expression of alkaline phosphatase, osteocalcin, osteonectin, ICAM-1, RUNX-2, and collagen 1a was assessed.
Results:
In osteoblast-like cells cultured with conditioned medium, the A-PRF+ conditioned medium induced more mineralization and calcium production after 28 days of culturing compared with the control (p < .05). No significant differences were found in the extent of cell proliferation between the different conditions. RUNX-2 and osteonectin mRNA expression in the cells were lower in all PRF-stimulated cultures compared with control at different time points. The i-PRF-conditioned medium induced more ALP activity (p < .05) compared with control and osteoblasts-like cells differentiated more compared with osteoblasts cultured with L-PRF.
Conclusions:
The three PRF preparations seem to have the capacity to increase the osteogenic potential of osteoblast-like cells. A-PRF+ seems to have the highest potential for mineralization, while i-PRF seems to have the potential to enhance early cell differentiation.
Further details about PRF using in dentistry you can find on our website.
1 INTRODUCTION
Loss of alveolar bone support is one of the characteristics of periodontal disease. This results in periodontal osseous lesions. The presence of periodontal osseous lesions is clinically significant in many ways. It relates to the associated loss of tooth support, to the site-specificity of periodontal destruction, and to the possibility that local factors (deep pockets and furcation involvement) associated with some osseous lesions may represent risk factors or indicators for disease progression. The surgical regeneration of the destructed periodontium may have multiple advantages, such as: (i) an increase in the periodontal attachment and bone of a severely compromised tooth; (ii) a decrease in pocket depth; and (iii) no, or a minimal, increase in gingival recession.
To achieve a successful periodontal tissue regeneration, different kinds of cells need to be targeted. Osteoblasts and osteoclasts are key cells in bone remodeling. In health, bone formation and bone resorption are in balance. To achieve bone regeneration, it is essential to stimulate osteoblasts for their role in bone formation. Platelet-rich fibrin (PRF) is one the materials that can guide different types of cells to promote tissue healing. Two of the main advantages of PRF include the fact that it contains living blood born cells such as leukocytes, intact platelets, and stem cells, which are important inflammatory cells and which play key roles in wound healing. Notably, they all release slowly and steadily growth factors and adhesion molecules for proper tissue repair.
PRF was initially developed with a high relative centrifugation force (RCF), allowing a dense fibrin clot to form.6 The standard procedure for the PRF is called L-PRF. Recently, new protocols for the preparation of PRF have been introduced. The advanced procedure (A-PRF+) is developed using a lower RCF. The generated A-PRF+ fibrin matrix shows a more porous structure, in which platelets and leucocytes remain trapped during the centrifugation phase. Further protocols are present in the literature as well. With the application of an even lower RCF, it is possible to modulate the coagulation cascade, obtaining a liquid material that can be used alone or mixed with other biomaterials before its complete clotting to promote tissue healing. The liquid form of PRF is called injectable PRF (i-PRF). The development and the applications of i-PRF have been pursued as an easy-to-use platelet concentrate in liquid form which can be either used alone or combined with various other biomaterials, such as allogenic and xenogenic bone substitutes. In this way, the biomaterials have an easier handling and more importantly are biologically active since it has been shown to contain high levels of growth factors, such as PDGF-AA, PDGF-AB, EGF, and IGF-1 which accelerate wound healing and regulate cell growth and division.
Recently, it was shown that L-PRF, A-PRF, and i-PRF have a stimulatory effect on the migration and proliferation of periodontal fibroblasts. Another study showed that L-PRF membranes induce a significant proliferation and differentiation of human osteoblasts and fibroblasts. i-PRF also increases the osteoblastic activity in vitro.1 However, to our k nowledge, there is no study that investigates and compares the differential effects of A-PRF+, L-PRF, and i-PRF on osteoblasts in vitro. Therefore, the aim of this study was to assess the osteogenesis potential of the three different PRF protocols (A-PRF+, L-PRF, i-PRF) on a human osteoblast-like cell line in vitro.
2 MATERIALS AND METHODS
2.1 PRF preparation
Blood samples were collected from six healthy male volunteers in accordance with the Medical Ethical Committee of VU Medical Center, Vrije Universiteit Amsterdam (study protocol reference 2016.530). After signing an informed consent regarding the procedure and the relative risks of it, six male, non-smoking, healthy volunteers donated blood for 2 A-PRF+ tubes (10 mL tube, glass, PRF Process), 2 L-PRF tubes (9 mL tube, glass-coated plastic tubes, Intra-Lock), and 2 i-PRF tubes (10 mL tube, plastic, PRF Process). The centrifugation process started for all the tubes in less than 1 min after the blood samples were collected. The protocols for the preparation of different PRF were described in Table 1, and followed the guidelines by Miron et al. For the A-PRF+ membranes, the tubes were placed in the Duo Quattro (Process for PRF) centrifuge with the rotor angulated at 40° and radius of 88 mm at the clot and 110 mm at the max. For the L-PRF membranes, the tubes were placed in the Intra-Lock (Intra-Lock) centrifuge with the rotor angulated at 33° and radius of 50 mm at the clot and 80 mm at the max. For i-PRF, the tubes were placed in the Duo Quattro (Process for PRF) centrifuge with the rotor angulated at 40° and radius of 88 mm at the clot level and 110 mm at the max. Times and RCF-max of the different preparations are shown in Table 1.
TABLE 1. Centrifugation protocols.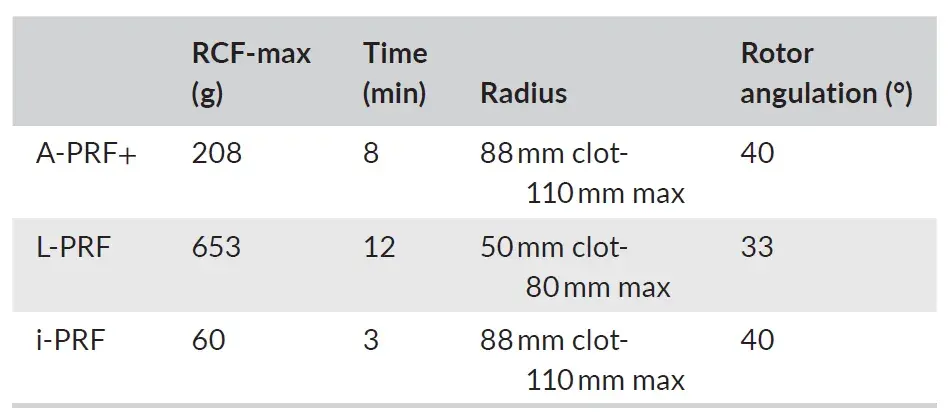
After centrifugation, the tubes of A-PRF+ and L-PRF were kept vertical for 5 min, during this time, the fibrin clot was formed in the middle of the tube. Sterile tweezers were used to take the fibrin clots out of the tube. Fibrin clots were separated from the acellular plasma using sterile scissors. Then the fibrin clots were placed in a sterile metal box device (Xpression Fabrication Kit, Intra-Lock). A homogenous pressure was applied for 5 min. Subsequently, the clots were removed from the metal box in sterile conditions (airflow cabinet) and were placed in 6-well plates (Greiner-Bio-One International GmbH). Each PRF membrane was placed in one well, containing 10 mL of α-MEM (Invitrogen Life Sciences), medium containing 5% FC1 (Fetal clone I, HyClone Laboratories) and 1% PSF (Antibiotics Penicillin/Streptomycin/Amphotericin B, Sigma-Aldrich). After the centrifugation of the i-PRF tube, the supernatant was extracted directly with a sterile pipette under sterile conditions (airflow cabinet) and was placed directly in wells containing 10 mL of α-MEM (Invitrogen Life Sciences), medium containing 5% FC1 (Fetal clone I, HyClone Laboratories) and 1% PSF (Antibiotics Penicillin/Streptomycin/Amphotericin B, Sigma-Aldrich).
A-and L-PRF membranes were incubated in above-mentioned medium for 6 days at 37°C and 5% CO2. To facilitate the release of growth factors, the plates were put on a rocking platform shaker (VWR, Batavia) with low speed and tilt (speed 1; tilt 3) in the incubator (HeraCell, Thermo Fisher Scientific Inc.). After 6 days, the conditioned media were extracted and stored at −80°C.
2.2 Osteoblast cell line culture
The osteoblast cell line U2OS (passage number 28) was seeded in 6-well plates 14 days prior to the experiment. Osteoblasts were cultured with α-MEM medium (Invitrogen Life Sciences), containing 10% FC1 (Fetal clone I, Hyclone Laboratories) and 1% PSF (Antibiotics Penicillin/Streptomycin/Amphotericin B, Sigma-Aldrich). The cells were then plated in a 24-well plate (Greiner-bio-one International GmbH) with 5 × 103 cells per well. Subsequently, 500 μL of conditioned medium from the different PRF conditions (A-PRF+, L-PRF, i-PRF) was added to the seeded osteoblasts. As a control, α-MEM (Invitrogen Life Sciences) medium containing 5% FC1 (Fetal clone I, HyClone Laboratories) and 1% PSF (Antibiotics Penicillin/Streptomycin/Amphotericin B, Sigma-Aldrich) was used. Media were refreshed twice a week. An additional 50 μg/mL ascorbic acid (Sigma-Aldrich) and 10 nM β-Glycerophosphate (Sigma-Aldrich), which are conducive to mineralization, were added twice a week to the well plates which were used for the mineralization assay and the calcium assay. The cells were cultured at 37°C and 5% CO2 (HeraCell, Thermo Fisher Scientific Inc.).
2.3 Real-time qPCR assessment
On days 0, 7, 14, and 21, RNA was extracted from the osteoblast cell-line cultures using an RNeasy Mini kit (Qiagen) according to the protocol proposed by the manufacturer. RNA concentration was measured with Synergy HT® spectrophotometer (BioTek Synergy HT, Beun de Ronde) at 260 and 280 nm. RNA was used in the reverse transcriptase reaction, which was performed according to the manufacturer's instructions of the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas). Oligo(dT) 18 and D(N)6 primers were used. The sequences of the DNA probes that were used in the q-PCR analysis are shown in Table 2. Primer Express software, version 2.0 (Applied Biosystems) was used to design the Real-time PCR primers (The primer sequences that were used are presented in Table 2). The expression was measured by real-time qPCR using the LC480 light cycler (Roche). 1 ng cDNA was used in a total volume of 20 μL containing Light Cycler SybrGreen1 Master mix (Roche) and 1 μM of each primer. Melting curve analysis was used to determine whether any unspecific PCR products were generated. The expression of the genes runt-related transcription factor 2 (RUNX-2), Osteocalcin (OC), Collagen 1a (Col-1A), Alkaline Phosphatase (ALP), Osteonectin (ON) and intercellular adhesion molecule 1 (ICAM-1) was investigated. Porphobilinogen deaminase (PBGD) was used as a housekeeping gene. The sample expression was normalized for PBGD expression by calculating DC (Ct, PBGD—Ct, gene of interest), where the xpression of the genes is calculated as 2-(δCt).
TABLE 2. Oligonucleotide sequences of the DNA probes that were used in the q-PCR analysis. Note: Housekeeping genes used as control, results are relative to PBGD expression. Abbreviations: ALP, alkaline phosphatase; COL1A, collagen 1; ICAM 1, intercellular adhesion molecule 1; OC, osteocalcin; ON, osteonectin; PBGD, porphobilinogen deaminase; RUNX2, runt-related transcription factor 2.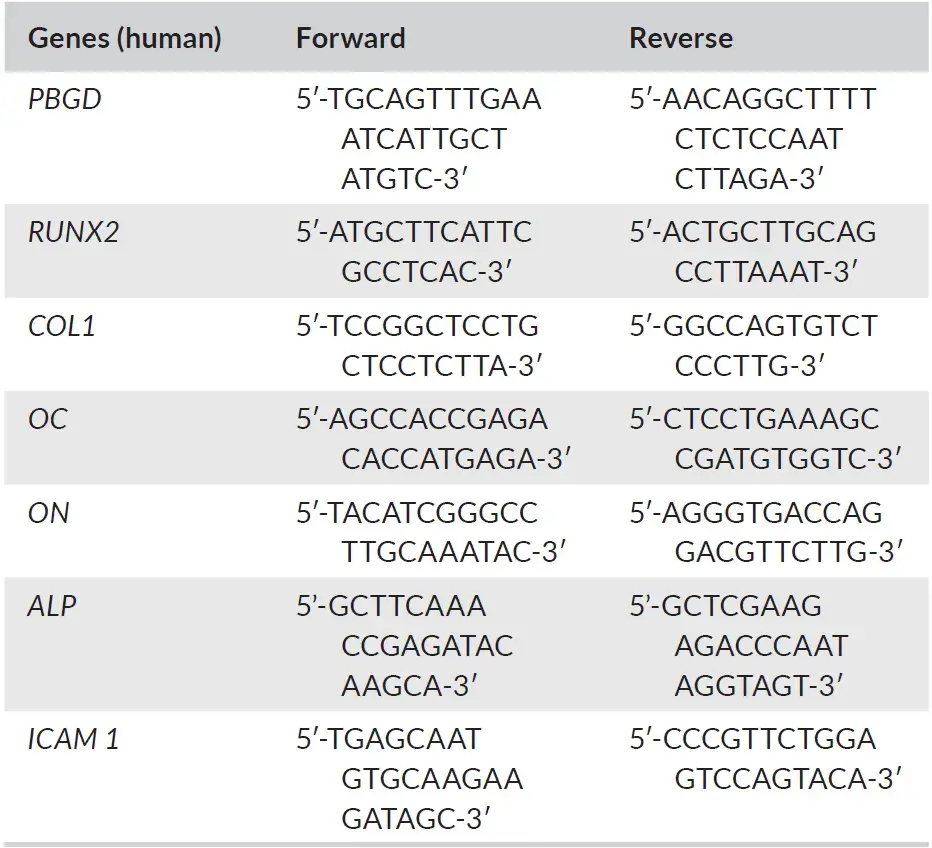
2.4 Calcium assay
Calcium assay was performed on day 28. 0.5 mL of 0.5 M of acetic acid was added to the wells. Extraction of calcium was accomplished by shaking samples for 1 day. The ortho-cresolphthalein complexone method (OCPC) was used to measure the calcium content.18 The OCPC solution consisted of 80 mg OCPC (Sigma-Aldrich) in 75 mL H2O with 0.5 mL 1 N KOH and 0.5 mL 0.5 M acetic acid. Subsequently, the working solution was made as follows: 5 mL OCPC solution to 5 mL of 14.8 M ethanolamine/boric acid buffer (pH = 11), 2 mL of 8-hydroxyquinoline, and 88 mL of MilliQ water. Finally, the freshly made 100 mL working solution was added to the 100 μL. Measurements were performed in Synergy HT® spectrophotometer (BioTek Synergy HT) at 570 nm.
2.5 Alkaline phosphatase activity
Alkaline phosphatase activity was measured at three different time points (0, 7, 14 days). At these time points, the cultures were stopped by removing the medium and by adding 50 μL of milli-Q water to each well. Then the plates were stored at −80°C until measurement. For measurement, 200 μL o f m illi-Q water was added to each well so that each well-contained 250 μL. The plates were freeze/thawed five times and subsequently, the samples were collected by scraping the bottom of each well with a pipette tip and subsequently transferred to Eppendorf tubes and centrifuged for 5 min at 10.000 rpm. 20 μL in duplicate of the supernatant was used for analysis. Alkaline phosphatase was measured according to the method described by Lowry. In short, a 4-nitrophenyl phosphate disodium salt (Merck) at pH 10.3 was used as a substrate for alkaline phosphatase. Absorbance was measured at 405 nm with a Synergy HT® spectrophotometer (BioTek Synergy HT). DNA was measured in the same lysate using CyQuant Cell Proliferation Assay Kit (Molecular Probes). Fluorescence was measured at 485 nm excitation and 528 nm emission with a Synergy HT® spectrophotometer (BioTek Synergy HT) microplate reader. Alkaline phosphatase was expressed as nmol/ng DNA.
Protein levels were also measured in the same lysate using a bicinchoninic acid (BCA) Protein Assay Kit according to the manufacturers' instructions (Pierce) and absorbance was read at 540 nm with Synergy HT® spectrophotometer (BioTek Synergy HT). Differentiation of the cells was expressed as DNA/protein (ng/ng).
2.6 Mineralization assay
Mineralization was assessed after 28 days of culturing. Cells were fixated first with 4% formaldehyde in PBS for 10 min. Then the wells were rinsed with milliQ water. Subsequently, 2% Alizarin Red S was added at pH 4.3 (Sigma-Aldrich) for 5 min at room temperature. Thereafter, cells were washed with milliQ water and then Alizarin Red S (Sigma-Aldrich) was again added. This was repeated three times and thereafter, the wells were kept under tap water. Red nodules were a sign of mineral deposition and were visualized with a converted microscope (Leica).
2.7 Statistical analysis
Raw data were generated at ACTA, Amsterdam, Department of Cell Biology. Derived data supporting the findings of this study are available from the corresponding author [LP] on request. For the statistical analyses and the graphic presentation of data, GraphPad (version 9.1.0) was used. A Kolmogorov–Smirnov test was performed to check the normality of the data. Data were non-normally distributed, therefore, non-parametric tests were used. A Kruskall–Wallis test with post hoc Dunn–Sidak correction for multiple comparisons was performed to assess differences in the calcium concentration, ALP activity, differentiation, proliferation, and the relative expression of genes. Box plots presenting the max, the min, and the interquartile range were used for the presentation of the data. Statistical significance was considered for p < .05%.
3 RESULTS
Six healthy male volunteers aged from 29 to 55 donated blood for the PRF preparation. None of them was a smoker and they all had a normal BMI (<25 kg/m2). No adverse reactions were observed during and after the blood sampling. PRF of comparable volume was obtained from all the blood tubes.
3.1 Only RUNX-2 and osteonectin are upregulated in the control group in qPCR analysis
At the end of the experiments, the cells were retrieved from the cultures in order to analyze the qPCR expression. The results of the qPCR are presented in Figure 1. Expression of runt-related transcription factor 2 (RUNX-2) was statistically significantly higher when cells were cultured with control medium compared with conditioned medium of A-PRF+ and L-PRF at day 7 (p < .01) and statistically significantly higher compared with A-PRF+ at day 14 (p < .05) (Figure 1A). Osteocalcin (OC) was significantly higher (p < .01) at day 21 compared with day 14 in the L-PRF group (Figure 1B). Moreover, OC w as u pregulated a t d ay 2 1 c ompared with day 7 (p < .05) in the i-PRF group (Figure 1B). There were no statistically significant differences in the expression of Collagen 1a (Col-1A) between different time points and different conditions (Figure 1C).
The expression of osteonectin (ON) was upregulated on day 7 in the control group. This was statistically significantly different compared with A-PRF+ and L-PRF at day 7 (Figure 1E). At 14 and 21 days, no differences were present between the different conditions and between the various time points in the same group.
The expression of intercellular adhesion molecule 1 (ICAM-1) was higher (Figure 1F) at day 21 compared with day 7 (p < .05) and at day 21 compared with day 14 (p < .01) in the i-PRF condition. In the A-PRF+ and L-PRF conditions, a tendency of upregulation at day 21 was present, however not statistically significant.
Alkaline phosphatase (ALP) expression in A-PRF+ at day 21 was significantly higher (p < 0.01) compared with day 7. ALP expression in L-PRF at day 21 and day 14 was significantly higher compared with day 7 with p < .01 and p < .05, respectively (Figure 1D).
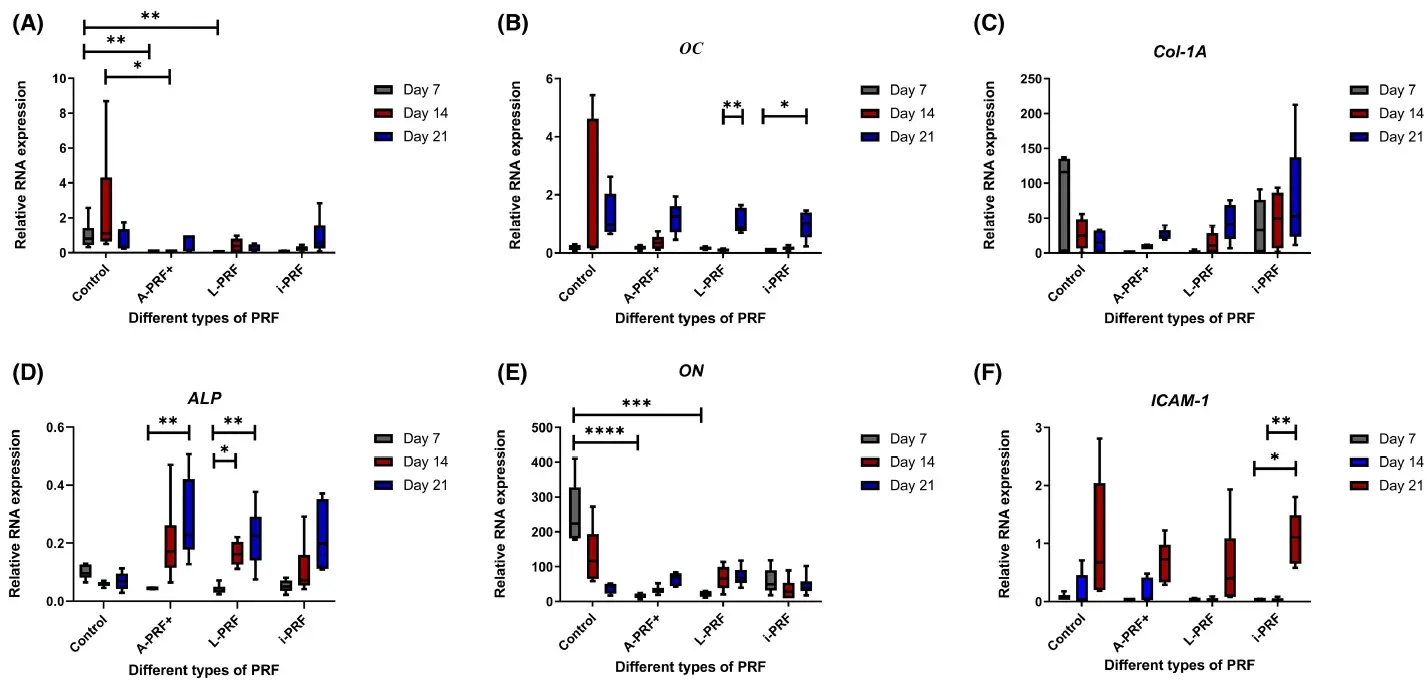 FIGURE 1. Relative expression of different osteogenic gene markers through a real-time qPCR analysis. (A) RUNX2: runt-related transcription factor 2, (B) OC: Osteocalcin, (C) Col-1A: Collagen 1a, (D) ALP: Alkaline Phosphatase, (E) ON: Osteonectin, (F) ICAM-1: intercellular adhesion molecule 1. Box plots are illustrating the max, the min, and the median. p < .05, *p < .01, ***p < .001, ****p < .0001.
FIGURE 1. Relative expression of different osteogenic gene markers through a real-time qPCR analysis. (A) RUNX2: runt-related transcription factor 2, (B) OC: Osteocalcin, (C) Col-1A: Collagen 1a, (D) ALP: Alkaline Phosphatase, (E) ON: Osteonectin, (F) ICAM-1: intercellular adhesion molecule 1. Box plots are illustrating the max, the min, and the median. p < .05, *p < .01, ***p < .001, ****p < .0001.
3.2 Cells cultured with A-PRF+ deposit more calcium
Calcium production was significantly higher in the cells cultured with conditioned medium derived from A-PRF+ compared with control (p < .05) (Figure 2). No differences were found between L-PRF, i-PRF, and control.
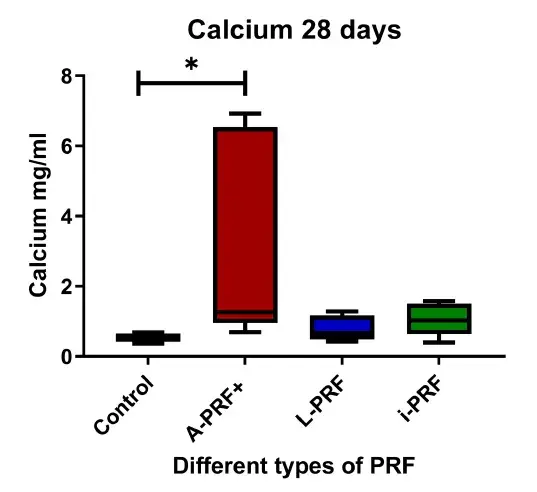 FIGURE 2. Calcium deposition after 28 days of culturing. Box plots are illustrating the max, the min interquartile range and the median. *p < .05.
FIGURE 2. Calcium deposition after 28 days of culturing. Box plots are illustrating the max, the min interquartile range and the median. *p < .05.
3.3 Cells cultured with i-PRF show higher alkaline phosphatase activity
The ALP activity and DNA content were measured in each well to express the ALP activity per DNA. We found that the ALP activity was upregulated when cultured with conditioned medium from i-PRF. There was a statistically significant difference between the i-PRF group and the control at day 7 (Figure 3A) and at day 14 (Figure 3B). ALP activity was also significantly higher in the i-PRF cultures compared with the A-PRF+ at day 14 (Figure 3B) (p < .05).
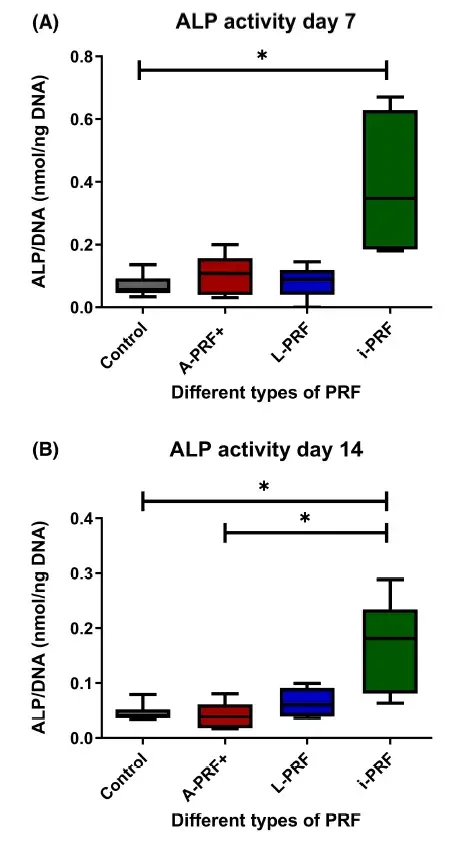 FIGURE 3. Alkaline phosphatase (ALP) activity. (A) Day 7 of culture. (B) Day 14 of culture. Box plots are illustrating the max, the min interquartile range and the median.*p < .05.
FIGURE 3. Alkaline phosphatase (ALP) activity. (A) Day 7 of culture. (B) Day 14 of culture. Box plots are illustrating the max, the min interquartile range and the median.*p < .05.
3.4 i-PRF- conditioned medium causes more differentiation than osteoblasts cultured with in conditioned medium from L-PRF
To investigate cell differentiation influenced by the various PRFs, the amount of protein and DNA was measured which can be used as a measure for differentiation. Osteoblasts cultured with conditioned medium derived from i-PRF differentiated more than osteoblasts cultured with conditioned medium derived from L-PRF at day 7 (Figure 4A). No other differences were found between the different conditions and time points (Figure 4).
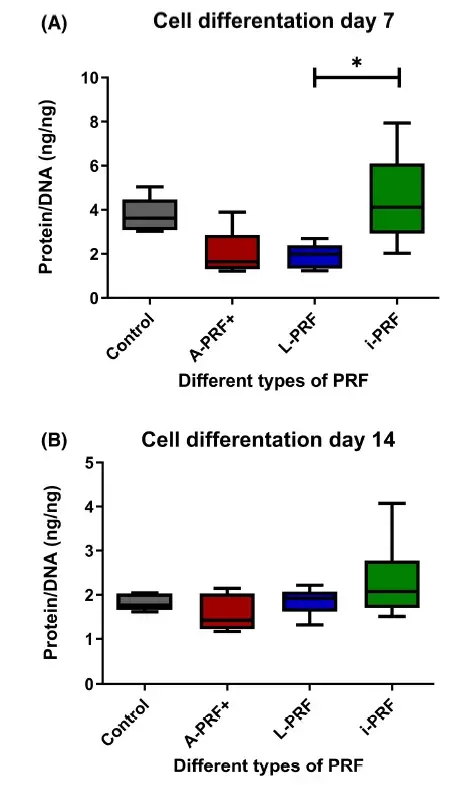 FIGURE 4. Cell differentiation. (A) Day 7 of culture. (B) Day 14 of culture. Box plots are illustrating the max, the min interquartile range and the median. *p < .05.
FIGURE 4. Cell differentiation. (A) Day 7 of culture. (B) Day 14 of culture. Box plots are illustrating the max, the min interquartile range and the median. *p < .05.
3.5 Different PRF conditions do not induce higher proliferation of osteoblasts
There was a significantly higher number of cells at 7 days and 14 days compared with day 0 (p < .05) in all groups (Figure 5). There were no statistically significant differences between the different conditions. Moreover, no statistically significant differences were found between the number of cells on days 7 and 14.
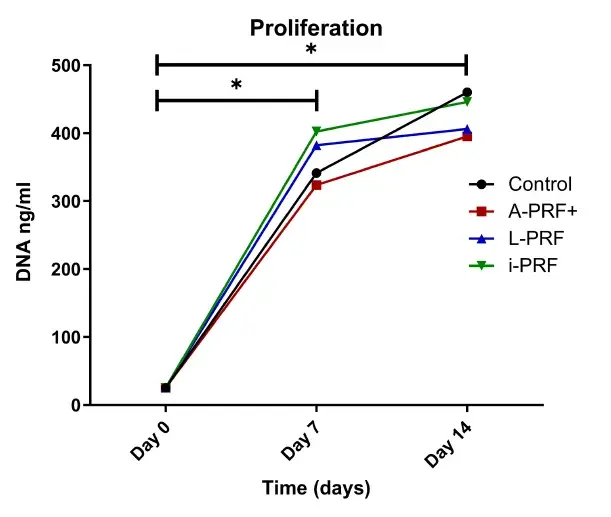 FIGURE 5. Proliferation after 7 and 14 days. The total amount of DNA content per well is illustrated. *p > .05.
FIGURE 5. Proliferation after 7 and 14 days. The total amount of DNA content per well is illustrated. *p > .05.
3.6 More mineralization was found in the cell cultures with conditioned medium derived from A-PRF+
A descriptive evaluation was performed by an experienced cell biologist (I.J.). The cell cultures with conditioned medium from A-PRF+ showed more mineralization compared with. Cultures with conditioned medium derived from L-PRF and i-PRF showed almost no staining for minerals. Hematoxylin–eosin staining showed that less cells were present in the L-PRF and i-PRF conditions. An example of three donors is shown in Figure 6.
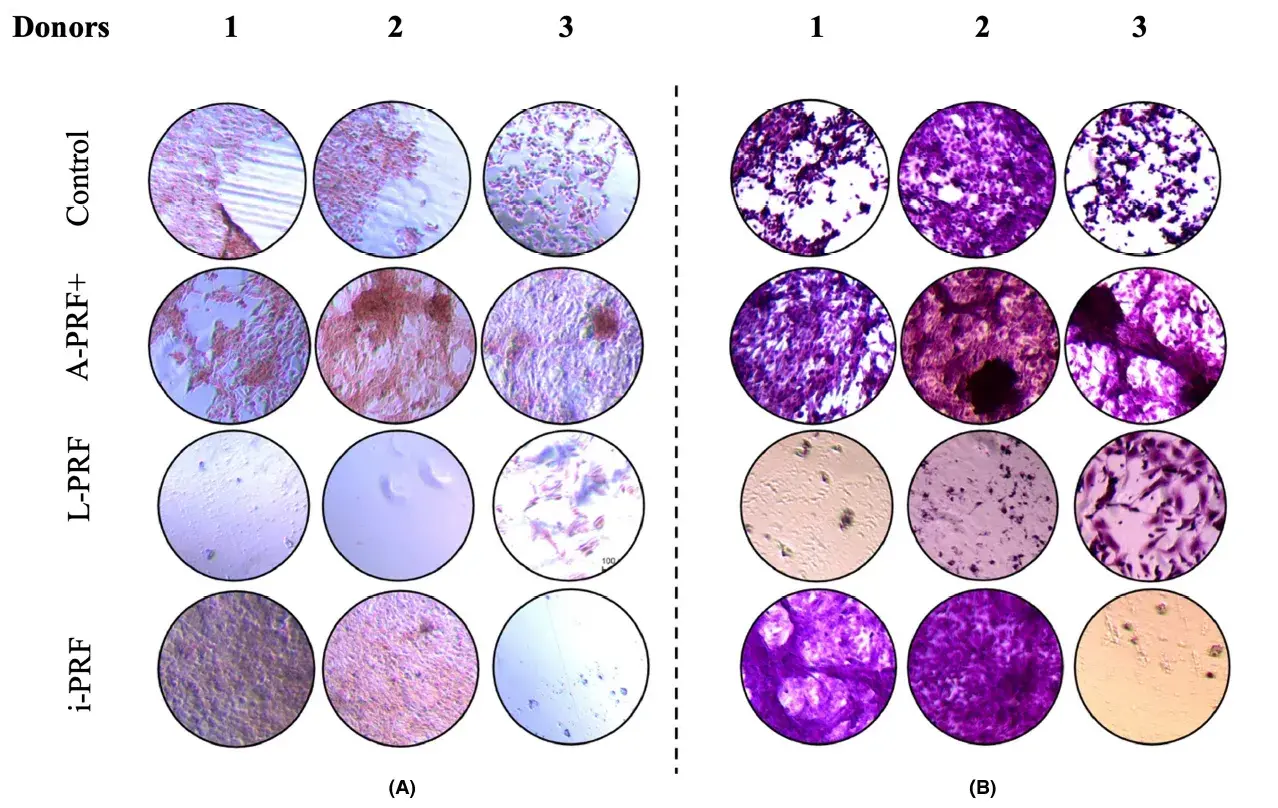 FIGURE 6. Hematoxylin–eosin staining (A) combined with alizarin red (B) staining at day 28 of culture. Image captured under optical microscope with a magnification. Note the decreased number of cells in plates L-PRF 1, 2, and i-PRF in plates 3. Example of cell cultures from three patients.
FIGURE 6. Hematoxylin–eosin staining (A) combined with alizarin red (B) staining at day 28 of culture. Image captured under optical microscope with a magnification. Note the decreased number of cells in plates L-PRF 1, 2, and i-PRF in plates 3. Example of cell cultures from three patients.
4 DISCUSSION
The aim of this study was to investigate the effect of three different PRF preparation protocols on an osteoblast-like cell line in vitro. We showed that conditioned medium of A-PRF+ resulted in increased mineral deposition after 28 days of culturing compared with the control cultures without any PRF-conditioned medium. Mineralization and calcium deposition is a sign that the cells differentiate to bone-forming cells. Thus, it seems that A-PRF+, more than other PRF preparations, can promote the functional differentiation of the osteoblast-like cells. An explanation for these favorable results could be that in A-PRF+, due to the different preparation protocol, the amount of growth factors is higher or more complex than for other PRF preparations. The chemokines and cytokines released by the entrapped leukocytes in the PRF membrane could promote the maturation of osteoblasts, resulting in elevated calcium levels and minerals deposition. Interestingly, cultures with conditioned medium from L-PRF and i-PRF showed minimal mineralization; visualization under the microscope and staining with hematoxylin/eosin showed that the number of osteoblast-like cells in the cultures with these latter two preparations was less compared with the other conditions (Figure 6). A possible explanation for this is that the cells cultured with L-PRF and i-PRF-conditioned medium proliferated much faster which results in overgrowth and subsequent detachment of the cells and the deposited mineral. Unfortunately, we have no data on cell numbers in the various cultures beyond day 14.
These observations are complex to interpret. Our findings are different from those of Wang et al. who showed that i-PRF induced the proliferation and the osteoblast differentiation of human primary osteoblasts significantly more at 3 and 5 days compared with the control groups. The different results may be due to other research designs and individual variations in the time intervals between blood sample centrifugation and cell culture treatment. We used in our study osteoblast-like cells from a cancer cell line, while they used primary human osteoblasts. As is known, cancer cells have their own metabolism and will undoubtedly have mutations in cell pathways that are usually targeted by large T antigens. In our study, we analyzed cell proliferation at days 7 and 14, while in the study by Wang et al., cell proliferation was analyzed at days 3 and 5. For future experiments, the differences in cell proliferation can be followed by shortening the time (for example, days 3, 5, and 7) of the cell culture so that there is no overgrowth of cells in the well-plates, which can cause cell death.
The enzymatic activity of the alkaline phosphatase is crucial in the process of the mineralization of the intercellular matrix by osteoblasts. We found that the osteoblasts cultured with i-PRF appear to have increased enzymatic activity of alkaline phosphatase compared with osteoblasts cultured without PRF-conditioned medium and osteoblasts cultured with A-PRF+ medium at 7 and 14 days. Moreover, it was shown that osteoblasts cultured with i-PRF medium differentiate more than the osteoblasts cultured with L-PRF medium. This is in accordance with previous research, in which it is shown that i-PRF increases the activity of alkaline phophatase promotes the differentiation of the osteoblasts. On the other hand, the ALP gene expression, which was analyzed in qPCR, was not significantly higher in the i-PRF condition. This indicates that i-PRF does not upregulate the expression of this gene but only induces the activity of the alkaline phosphatase.
The proliferation of osteoblasts in all conditions increased significantly in the first 7 days. On day 14, the number of cells was increased but not significantly compared with day 7. As expected, when the osteoblasts start to differentiate, the proliferation of the cells is downregulated. Furthermore, we showed that no condition resulted in an increased proliferation rate of the osteoblasts. This is in contrast with the results presented in a previous study, however, in that study, primary human osteoblasts were used and not a cell line.
Various findings on the gene expressions will be discussed. The qPCR analysis showed that the expression of RUNX-2 was significantly higher in the control group compared with the A-PRF+ and the L-PRF group. But in all the PRF groups, the expression was increased with increasing culture time, but this was not significant. It is known that RUNX-2 is an early marker of osteoblast differentiation and chondrocyte maturation and has the highest expression at day 7. During osteoblast differentiation, RUNX-2 is weakly expressed in mesenchymal cells, and its expression is upregulated in preosteoblasts, reaches the maximal level in immature osteoblasts,and is downregulated in mature osteoblasts. Based on this knowledge, we can say that the differentiation of pre-osteoblasts to mature osteoblasts was higher compared with the control in the cultures with conditioned medium derived from A-PRF+ and L-PRF. An unexpected result was the high mRNA expression of ON in the control group in the first 2 weeks. Osteonectin is a non-collagenous protein of the bone matrix. Paola Ciceri et al found that the peak of osteonectin was simultaneous with calcium deposition on day 7, suggesting that osteonectin plays a potential role as a pro-calcifying factor. It was shown that the expression of this gene was lower in PRF conditions compared with control, indicating that factors present in all PRF conditions inhibit the expression of this gene in the first days. COL1-A is considered a late osteogenic marker. The role of collagen formation is crucial for the mineralization process. The highest expression of this gene is after 3 weeks. However, no statistically significant differences could be found in the different conditions for this gene, although a tendency for increased expression was present for all PRF conditions and not in the control condition. ICAM-1 is an important adhesionmolecule for osteoblasts. It is considered a late osteogenic marker. A clear upregulation was found during culture time for all conditions, also in the control. However, this was only significant for i-PRF at the various time points. OC was low on culture day 7. From literature, it is known that it first appears after 8–11 days of culture and its expression is connected with the formation of mineralized tissues. In all conditions, an upregulation was found during culture time, but only significant in L-PRF and i-PRF group at day 21. In an attempt to summarize the findings for the various gene expressions in different PRF conditions, we can conclude that RUNX-2 indicates higher number of mature osteoblasts in A-PRF+ and L-PRF conditions, while the differences in ON indicate that at an early phase, PRF conditions may slow down the calcificationprocess. For COL1-A, ALP and ICAM-1, we did not find statistically significant differences between conditions. It seems that the expression of the latter genes remains the same after culturing with conditioned medium derived from different PRF protocols.
One of the strengths of this study is the relatively long observation period (up to 28 days) with various assays and time points to detect possible differences in osteogenesis such as relative expression of different genes with qPCR analysis, mineralization assay, calcium assay, alkaline phosphatase assay, proliferation, and differentiation. This allowed us to investigate both the short and long-term effects. For this reason, we chose to analyze very early markers like RUNX-2, early markers like ALP, and late markers like OC and COL-1A; so we could compare the different timepoints. Another strength of the study is the in vitro comparison of three different widely used PRF protocols (A-PRF+, L-PRF, i-PRF). These are the most widely used PRF preparations in the dental field in the last years, especially for guided bone and guided tissue regeneration31 and are applied to accelerate wound healing. Osteoblasts are the main cells involved in guided bone regeneration and they play a key role in the guided tissue regeneration together with the PDL fibroblasts. There are many in vitro studies showing the effect of PRF in PDL cells and gingival fibroblasts, however, there were no comparative studies of the effects of these three protocols on osteoblasts till now. A further advantage of the current study is the fact that all the preparations of the different PRFs were performed in parallel and specific attention was given to the timing of the centrifugation, which can play a significant role in the characteristics of the fibrin clot.
One of the limitations of this study is that we did not use the fibrin matrixes themselves in cell cultures. We used the conditioned medium, which contains the growth factors released after culture of the fibrin matrixes for 6 days. According to the literature, most of the growth factors present in the PRF matrix are released in this period. The reason that we used this protocol is that we can study the effect of soluble growth factors and other released molecules, as well as that the fibrin clots contain also leukocytes and other immune cells from the donors; the latter could lead to a cross-reaction between the donor-leukocytes and the osteoblast cell line, which can also influence the results. However, clinically, the fibrin matrix itself applied as autograft can play an important role in osteogenesis since it can function as a scaffold for the cells. Another limitation of this study is that an osteoblast-like cell line was used and not osteoblasts derived from the alveolar bone of the PRF donors. In our study, we decided to use only healthy donors, so no intervention in the mouth to retrieve osteoblasts of the alveolar bone could be ethically justified. Although the use of osteoblast-like cell lines is a well-established method to evaluate osteogenesis, there is a possibility that this cell line which originates from bone osteosarcoma reacts differently to the PRFs than osteoblasts isolated from the alveolar bone of the same donor. In contrast, a previous study in human bone mesenchymal stem cells and not in a cell line, shows that L-PRF promotes significant proliferation and differentiation.
A further consideration is necessary. PRF technology is getting constantly updated, and possibly improved. Therefore, it will be of value to compare our results with some newer preparation techniques. Worth mentioning are the Titanium-PRF and the horizontal centrifugation, which according to the authors produces a PRF matrix that is richer in platelets and leukocytes compared with other PRF preparation methods. This is due to the slower and more gentle centrifugation process, which minimizes the destruction of platelets and leukocytes in the blood sample and may guarantee a better distribution of them in the PRF matrix. Nevertheless, the optimal PRF preparation method may depend on the specific research question and experimental setting.38 In our work, we used PRF preparation methods that have been well established and validated through previous research, providing a reliable baseline for comparison with newer methods.
To conclude, PRF preparations seem to have the capacity to increase the osteogenic potential of osteoblast-like cells. A-PRF+ seems to have the highest potential for bone mineralization while i-PRF seems to induce an earlier differentiation. Based on our research, we could indicate that A-PRF+ would be the most suitable for a clinical application that requires hard tissue regeneration; however, clinical studies comparing the three different PRF preparation protocols need to be performed to investigate whether there is a clinically relevant difference between the three types of PRF preparation.
There are additional information about PRF using during sinus lift surgery you can gain on our webinar "Advanced surgery on sinus lift" by Mauro Gebran.
List of authors:
Konstntinos Kosmidis
Karishma Ehsan
Luciano Pitzurra
Bruno Loos
Ineke Jansen
References
1. Papapanou PN, Tonetti MS. Diagnosis and epidemiology of periodontal osseous lesions. Periodontol 2000. 2000;2000(22):8-21.
2. Cortellini P, Tonetti MS. Clinical concepts for regenerative therapy in intrabony defects. Periodontol 2000. 2015;68(1):282-307.
3. Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: le PRF. Implant Dent. 2001;42(55):e62.
4. Castro AB, Herrero ER, Slomka V, Pinto N, Teughels W, Quirynen M. Antimicrobial capacity of leucocyte-and platelet rich fibrin against periodontal pathogens. Sci Rep. 2019;9(1):8188.
5. Davis VL, Abukabda AB, Radio NM, et al. Platelet-rich preparations to improve healing. Part II: platelet activation and enrichment, leukocyte inclusion, and other selection criteria. J Oral Implantol. 2014;40(4):511-521.
6. Marrelli M, Tatullo M. Influence of PRF in the healing of bone and gingival tissues. Clinical and histological evaluations. Eur Rev Med Pharmacol Sci. 2013;17(14):1958-1962.
7. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37-e44.
8. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45-e50.
9. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e51-e55.
10. Hermida-Nogueira L, Blanco J, García Á. Secretome profile of leukocyte-platelet- rich fibrin (L-PRF) membranes. Methods Mol Biol. 2023;2628:207-219.
11. Ghanaati S, Booms P, Orlowska A, et al. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40(6):679-689.
12. Miron RJ, Fujioka-Kobayashi M, Hernandez M, et al. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig. 2017;21(8):2619-2627.
13. Pitzurra L, Jansen IDC, de Vries TJ, Hoogenkamp MA, Loos BG. Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments. J Periodontal Res. 2020;55(2):287-295.
14. Ehrenfest DMD, Diss A, Odin G, Doglioli P, Hippolyte M-P, Charrier J-B. In vitro effects of Choukroun's PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):341-352.
15. Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets. 2018;29(1):48-55.
16. Miron RJ, Pinto NR, Quirynen M, Ghanaati S. Standardization of relative centrifugal forces in studies related to platelet-rich fibrin. J Periodontol. 2019;90(8):817-820.
17. Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20:2353-2360.
18. Connerty HV, Briggs AR. Determination of serum calcium by means of orthocresolphthalein complexone. Am J Clin Pathol. 1966;45:290-296.
19. Lowry OH. [17] Micromethods for the assay of enzymes. 1957.
20. Lian JB, Stein GS. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995;15:118-140.
21. Stein GS, Lian JB. Molecular mechanisms mediating developmental and hormone-regulated expression of genes in osteoblasts: an integrated relationship of cell growth and differentiation. Cellular and Molecular Biology of Bone. Elsevier; 1993:47-95.
22. Ahuja D, Sáenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24(52):7729-7745.
23. Sugawara Y, Suzuki K, Koshikawa M, Ando M, Iida J. Necessity of enzymatic activity of alkaline phosphatase for mineralization of osteoblastic cells. Jpn J Pharmacol. 2002;88(3):262-269.
24. Wang J, Li W, He X, Li S, Pan H, Yin L. Injectable platelet-rich fibrin positively regulates osteogenic differentiation of stem cells from implant hole via the ERK1/2 pathway. Platelets. 2023;34(1):2159020.
25. Dohan Ehrenfest DM, Doglioli P, de Peppo GM, Del Corso M, Charrier JB. Choukroun's platelet-rich fibrin (PRF) stimulates in vitro proliferation and differentiation of human oral bone mesenchymal stem cell in a dose-dependent way. Arch Oral Biol. 2010;55(3): 185-194.
26. Ciceri P, Elli F, Cappelletti L, et al. Osteonectin (SPARC) expression in vascular calcification: In vitro and ex vivo studies. Calcif Tissue Int. 2016;99(5):472-480.
27. Rossert J, Terraz C, Dupont S. Regulation of type I collagen genes expression. Nephrol Dialysis Transplant. 2000;15:66-68.
28. Karsenty G, Park R-W. Regulation of type I collagen genes expression. Int Rev Immunol. 1995;12(2–4): 177-185.
29. Tanaka Y, Morimoto I, Nakano Y, et al. Osteoblasts are regulated by the cellular adhesion through ICAM-1 and VCAM-1. J Bone Miner Res. 1995;10(10):1462-1469.
30. Hauschka PV, Reid ML. Timed appearance of a calcium-binding protein containing γ-carboxyglutamic acid in developing chick bone. Dev Biol. 1978;65(2):426-434.
31. Miron RJ, Zucchelli G, Pikos MA, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21(6):1913-1927.
32. Femminella B, Iaconi MC, Di Tullio M, et al. Clinical comparison of platelet-rich fibrin and a gelatin sponge in the Management of Palatal Wounds after epithelialized free gingival graft harvest: a randomized clinical trial. J Periodontol. 2016;87(2):103-113.
33. Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ. Behavior of gingival fibroblasts on titanium implant surfaces in combination with either injectable-PRF or PRP. Int J Mol Sci. 2017;18(2).
34. Castro AB, Andrade C, Li X, Pinto N, Teughels W, Quirynen M. Impact of g force and timing on the characteristics of platelet-rich fibrin matrices. Sci Rep. 2021;11(1):6038.
35. Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig. 2019;23(5):2179-2185.
36. Miron RJ, Chai J, Zheng S, Feng M, Sculean A, Zhang Y. A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A. 2019;107(10):2257-2271.
37. Uzun BC, Ercan E, Tunalı M. Effectiveness and predictability of titanium-prepared platelet-rich fibrin for the management of multiple gingival recessions. Clin Oral Investig. 2018;22(3):1345-1354.
38. Al-Badran A, Bierbaum S, Wolf-Brandstetter C. Does the choice of preparation protocol for platelet-rich fibrin have consequences for healing and alveolar ridge preservation after tooth extraction? A meta-analysis. J Oral Maxillofacial Surg. 2023.


